Alternatived Products of [ 826-81-3 ]
Product Details of [ 826-81-3 ]
| CAS No. : | 826-81-3 |
MDL No. : | MFCD00006765 |
| Formula : |
C10H9NO
|
Boiling Point : |
No data available |
| Linear Structure Formula : | H3CC9H5N(OH) |
InChI Key : | NBYLBWHHTUWMER-UHFFFAOYSA-N |
| M.W : |
159.18
|
Pubchem ID : | 13224 |
| Synonyms : |
|
Application In Synthesis of [ 826-81-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 826-81-3 ]
- 1
-
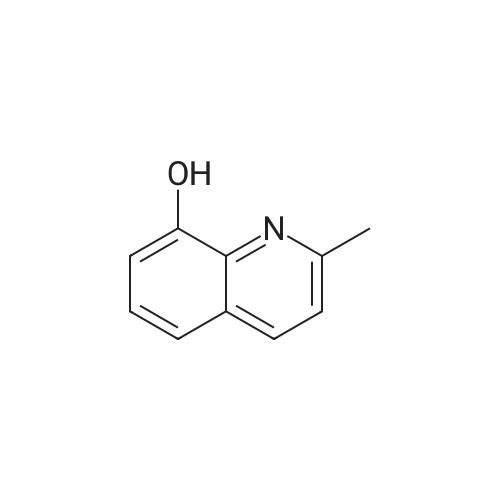 [ 826-81-3 ]
[ 826-81-3 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
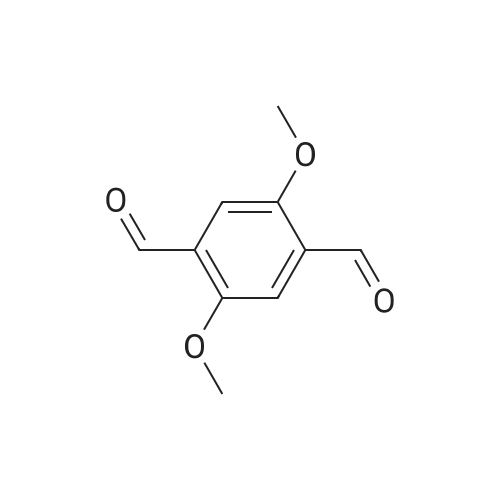 [ 7310-97-6 ]
[ 7310-97-6 ]

-
2,2'-(2,5-dimethoxy-1,4-phenylenedivinylene)bis-8-acetoxy quinoline
[ No CAS ]
- 2
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
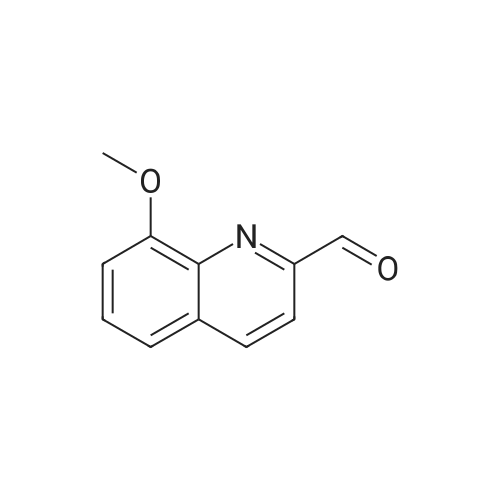 [ 103854-64-4 ]
[ 103854-64-4 ]
Reference:
[1]New Journal of Chemistry,2007,vol. 31,p. 927 - 935
[2]Patent: CN105294685,2016,A
[3]Patent: CN105418605,2016,A
[4]Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,p. 3376 - 3380
[5]RSC Advances,2016,vol. 6,p. 30405 - 30411
[6]ACS Chemical Neuroscience,2020,vol. 11,p. 4254 - 4261
- 3
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
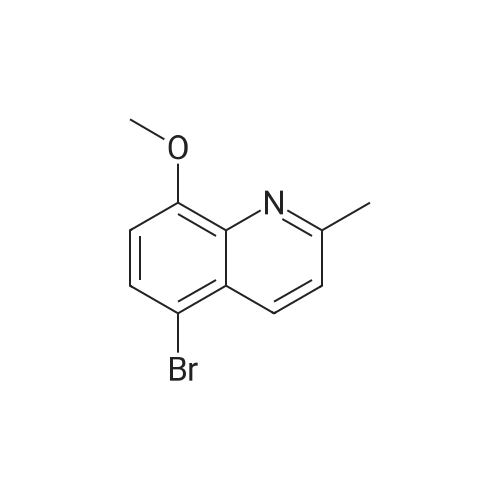 [ 103862-55-1 ]
[ 103862-55-1 ]
- 4
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
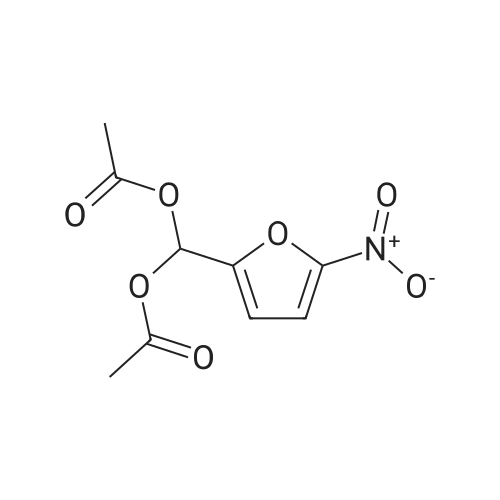 [ 92-55-7 ]
[ 92-55-7 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
C17H12N2O5
[ No CAS ]
- 5
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
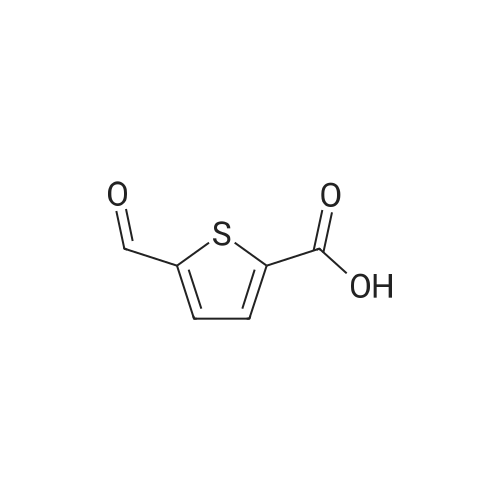 [ 4565-31-5 ]
[ 4565-31-5 ]

-
 [ 1131003-67-2 ]
[ 1131003-67-2 ]
- 6
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
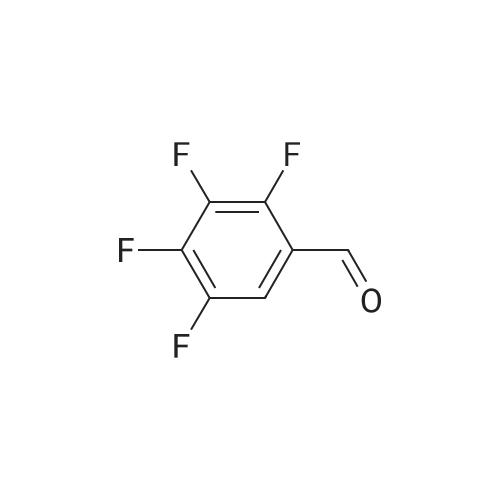 [ 16583-06-5 ]
[ 16583-06-5 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
(E)-2-[(2,3,4,5-tetrafluorophenyl)ethenyl]-8-acetoxyquinoline
[ No CAS ]
- 7
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
 [ 16583-06-5 ]
[ 16583-06-5 ]

-
(E)-2-[(2,3,4,5-tetrafluorophenyl)ethenyl]-8-hydroxyquinoline
[ No CAS ]
- 8
-
 [ 18826-95-4 ]
[ 18826-95-4 ]

-
 [ 95-55-6 ]
[ 95-55-6 ]

-
 [ 826-81-3 ]
[ 826-81-3 ]

-
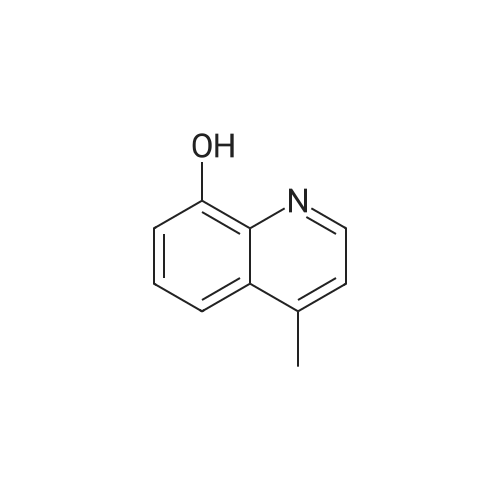 [ 3846-73-9 ]
[ 3846-73-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With iron(III) chloride hexahydrate; In tetrachloromethane; at 150℃; for 8.0h;Inert atmosphere; |
General procedure: A vial charged with 0.02 mmol of FeCl3·6H2O [FeCl3, FeCl2·4H2O,Fe(C5H5)2, Fe(acac)3, Fe(OAc)2, Fe2(CO)9], 2 mmol of aniline 1, 4 mmol of carbon tetrachloride and 8 mmol of 1,3-diol under argon was sealed, placed into a pressure reactor, and heated at 150C with stirring for 8 h. After the reaction completed the mixture was dissolved in hydrochloric acid, and separated. The aqueous layer was neutralized with 10% sodium hydroxide and extracted with methylene chloride. The organic layer was filtered and evaporated. The residue was distilled in a vacuum. Analytically pure samples were isolated by semi-preparative HPLC. Physical and chemical constants and spectral characteristics corresponded to those reported in [5, 6, 8-22]. |
- 9
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
 [ 92-55-7 ]
[ 92-55-7 ]

-
 [ 1204093-07-1 ]
[ 1204093-07-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 50% |
In acetic anhydride; at 150℃; for 30h; |
A solution of 0.8 g (5 mmol) of 8-hydroxy-2-methylquinoline, 4.87 g (20 mmol) of <strong>[92-55-7](5-nitrofuran-2-yl)methylene diacetate</strong> and 150 ml of acetic anhydride, the mixture was heated at 150 C for 30 hours (monitored by thin layer chromatography). The mixture was cooled and concentrated in vacuo to remove the solvent to obtain a crude product which was subsequently dissolved in pyridine / water (4: 1 by volume) solution at 100 C for 1 hour(monitored by thin layer chromatography). After the mixture was cooled, concentration in vacuo to remove solvent afforded a crude product, the residue was purified by column chromatography (FC, silica gel, methanol: dichloromethane = 1: 20), (E)-2-[2-(5-nitrofuran-2-yl)ethenyl]-8-hydroxyquinoline (Compound 11, 0.75 g, 50% yield). |
- 10
-
 [ 826-81-3 ]
[ 826-81-3 ]

-
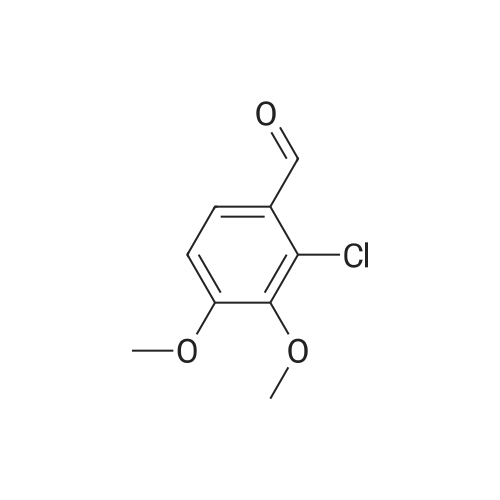 [ 5417-17-4 ]
[ 5417-17-4 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
(E)-2-[(2-chloro-3,4-dimethoxy)ethenyl]-8-acetoxyquinoline
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
for 20h;Reflux; |
General procedure: To a solution of 8-hydroxyquinaldine (10.0mmol) in acetic anhydride (10mL) was added the corresponding aldehyde (11.0mmol). The mixture was heated under reflux for 20h. After the mixture was cooled, it was subsequently poured into ice water (200mL) and stirred overnight. The solid obtained was filtered and washed with water. The residue was purified by silica-gel column chromatography using ethyl acetate/petroleum as eluent. A was obtained as a solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping















