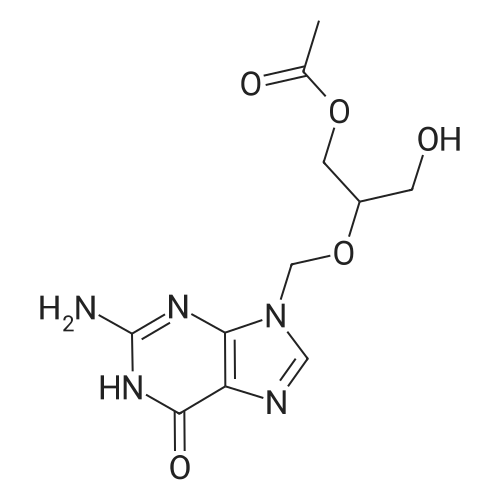
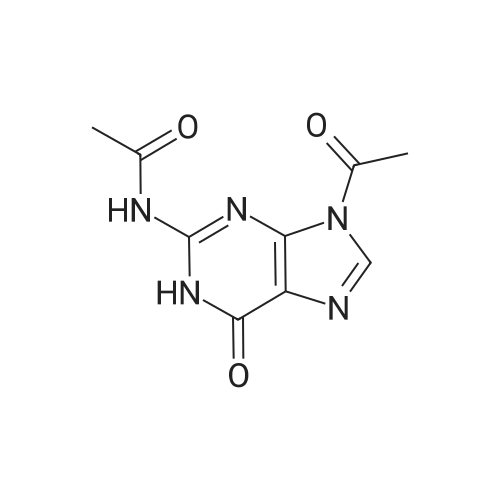
| 75.7% |
Stage #1: With Trimethyl borate In toluene for 5 h; Reflux; Large scale
Stage #2: at 20℃; for 6 h; Large scale
Stage #3: at 0 - 20℃; for 2 h; Large scale |
500.0 g (1.96 mol, 1.0 eq) of ganciclovir, 244.4 g (2.35 mol, 1.2 eq) of trimethyl borate,7.5 kg of toluene was added to the reaction vessel, and heated to reflux for 5 hours. The TLC controlled raw material reaction was complete.The temperature was lowered to room temperature, 396.7 g (3.93 mol, 2.0 eq) of triethylamine was added, then 323.7 g (2.94 mol, 1.5 eq) of 1-acetylimidazole was added, and the mixture was stirred at room temperature for 6 hours.The ratio of monoacetyl ganciclovirand N,O-diacetyl ganciclovir in the HPLC was 94/4.The temperature was lowered to 0 to 10 ° C, 2.0 kg of methanol was added dropwise, and the mixture was stirred at room temperature for 2 hours, and the solvent was concentrated under reduced pressure.Add 3.0 kg of ethyl acetate, wash once with 500 g of water, layer, and extract the aqueous layer with 500 g of ethyl acetate.Concentration under reduced pressure, ethanol recrystallization to give colorless crystals 440.9 g, yield 75.7percent, purity 99.2percent, |
| 75.7% |
Stage #1: With Trimethyl borate In toluene for 5 h; Reflux
Stage #2: With triethylamine In toluene at 20℃; for 6 h;
Stage #3: With methanol In toluene at 0 - 20℃; for 2 h; |
500.0g (1.96mol, 1.0eq) of ganciclovir, 244. 4g of trimethyl borate (2·35mol, 1 · 2eq), 7.5kg of toluene were added to the reaction kettle, heated to reflux for 5 hours, controlled by TLC The reaction of the starting material was completed, and the temperature was lowered to room temperature. Triethylamine 396 · 7 g (3 · 93 mol, 2.0 eq) was added, then 1 - acetylimidazole 323 · 7 g (2 · 94 mol, 1.5 eq) was added, and stirred at room temperature for 6 hours, HPLC The ratio of controlled monoacetyl ganciclovir and N,0-diacetyl ganciclovir was 94/4, the temperature was lowered by 0~10 ° C, 2.0 kg of methanol was added dropwise, and the mixture was stirred at room temperature for 2 hours, and concentrated under reduced pressure. the solvent, ethyl acetate was added 3.0kg, 500g once with water, separated and the aqueous layer was extracted with 500g ethyl acetate, the organic layers were combined and concentrated under reduced pressure, and recrystallized from ethanol to give colorless crystals 440.9g, a yield of 75.7percent, Purity 99.2percent |
| 75.7% |
Stage #1: With Trimethyl borate In toluene for 5 h; Reflux
Stage #2: With triethylamine In toluene at 20℃; for 6 h;
Stage #3: With methanol In toluene at 0 - 20℃; for 2 h; |
500.0 g (1.96 mol, 1.0 eq) of ganciclovir, 244.4 g (2.35 mol, 1.2 eq) of trimethyl borate,7.5 kg of toluene was added to the reaction kettle.Heated to reflux for 5 hours,The TLC controlled raw material reaction is complete,Down to room temperature,396.7 g (3.93 mol, 2.0 eq) of triethylamine was added, followed by the addition of 1-acetylimidazole 323.7 g (2.94 mol, 1.5 eq).Stir at room temperature for 6 hours.The ratio of monoacetyl ganciclovir and N,O-diacetyl ganciclovir in the HPLC was 94/4, the temperature was lowered by 0-10 °C, 2.0 kg of methanol was added dropwise, and the dropping was completed.Stir at room temperature for 2 hours.The solvent was concentrated under reduced pressure, and 3.0 kg of ethyl acetate was added, washed once with 500 g of water, and layered.The aqueous layer was extracted with EtOAc (EtOAc).Recrystallization of ethanol gave 440.9 g of colorless crystals.The yield is 75.7percent, the purity is 99.2percent, |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping