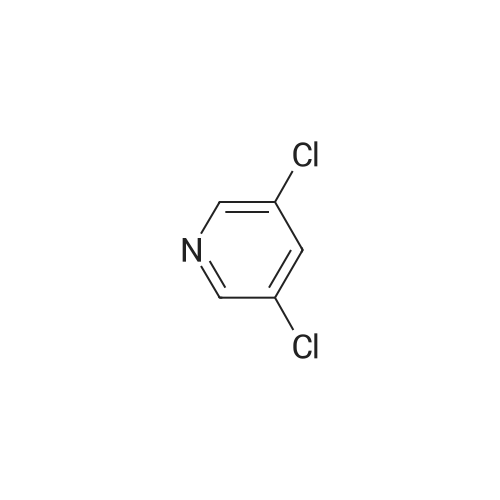
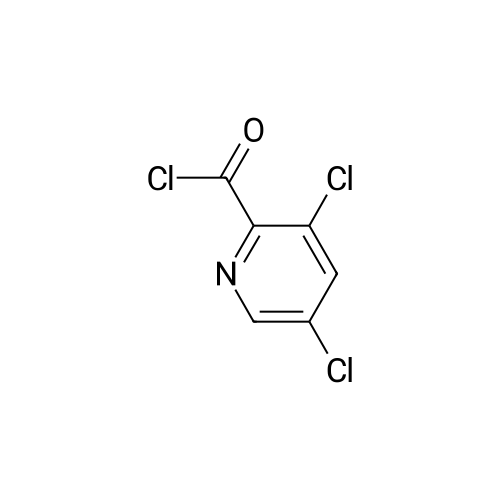
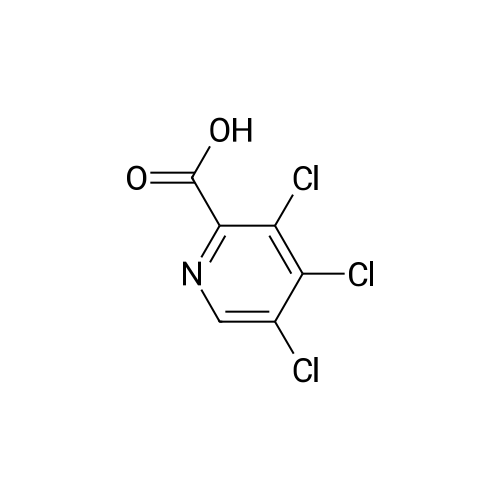
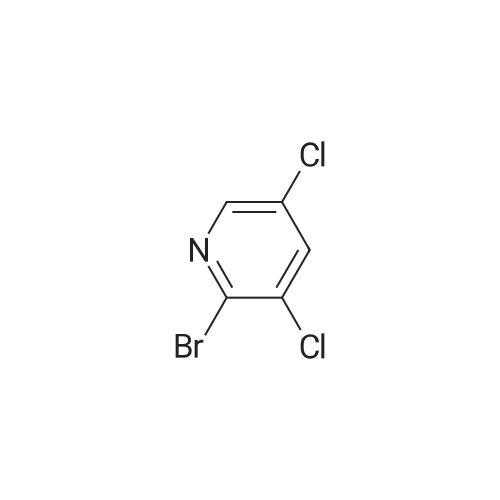
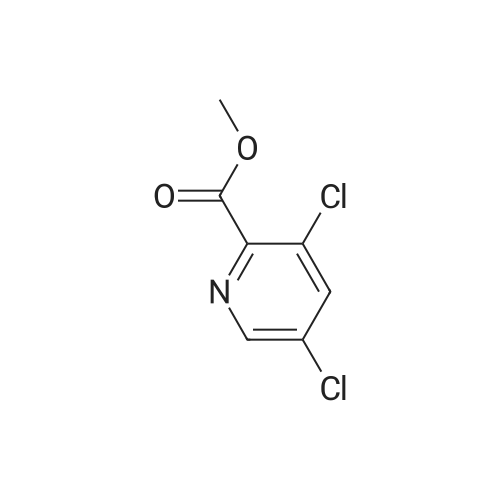
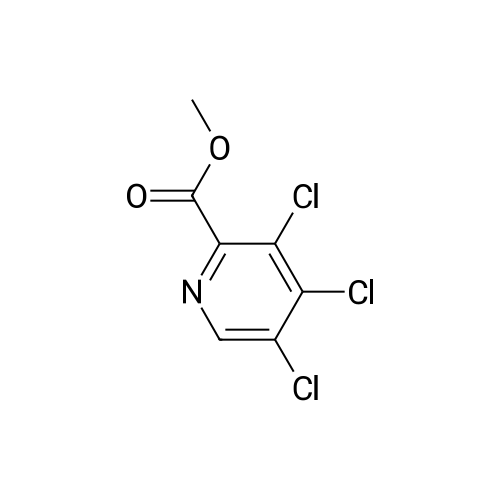
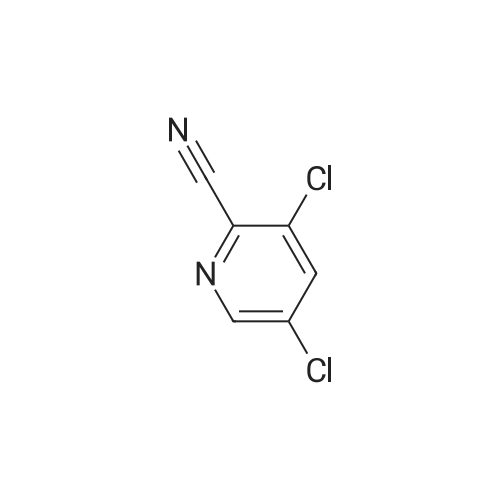
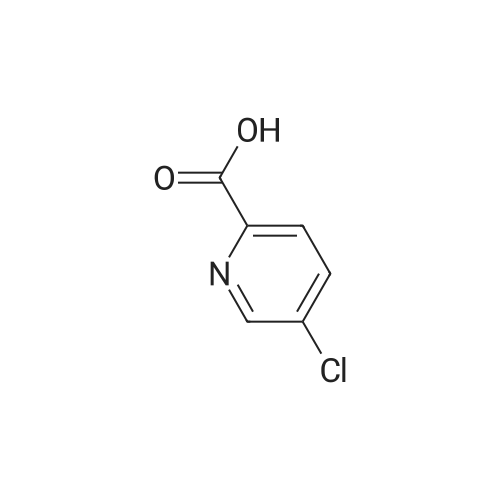
|
With thionyl chloride;N,N-dimethyl-formamide; for 0.25h;Heating / reflux; |
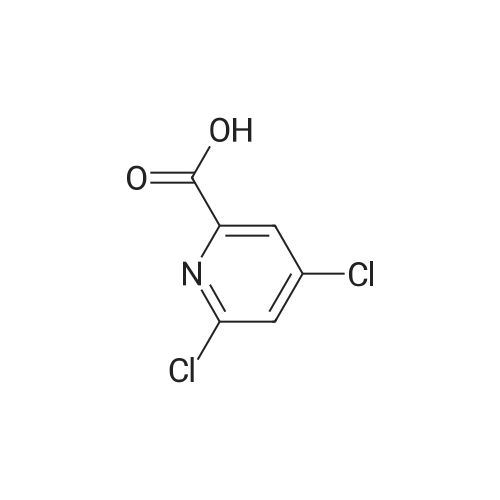
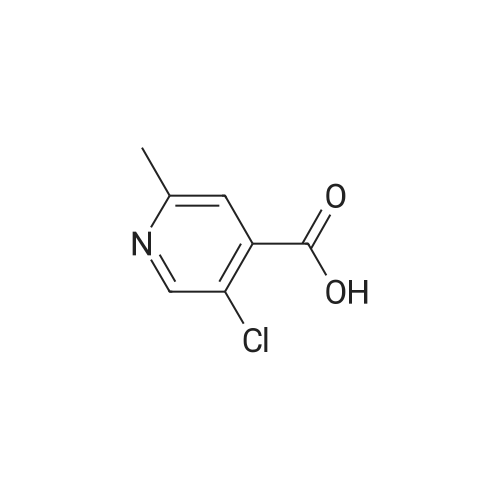
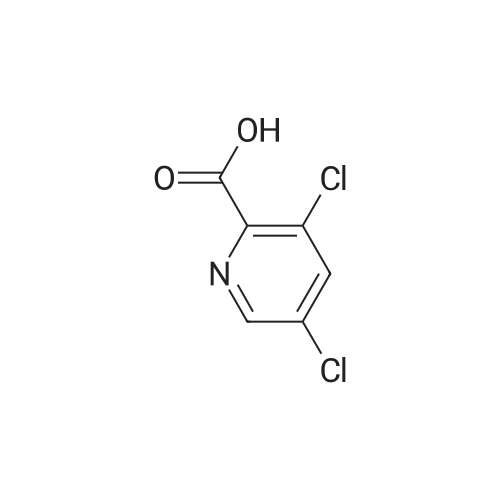
Example 54(a) 3,5-Dichloro-2-(piperidin-l-ylcarbonyl)pyridine; 3,5-Dichloro-2-pyridine carboxylic acid (1.25 g, 6.5 mmol) was suspended in thionyl chloride (10 ml). DMF (2 drops) was added and the mixture was refluxed for 15 minutes under an atmosphere of nitrogen. The solvent was evaporated. Toluene was added and the solvent was evaporated to give a solid. The solid was dissolved in DCM (8 ml) and the mixture was cooled to 0 C. Piperidine (0.64 ml, 6.5 mmol) was added dropwise followed by triethylamine (0.91 ml, 6.5 mmol). The cooling bath was removed. The mixture was <n="76"/>stirred under nitrogen atmosphere until RT was reached and then for an additional 15 minutes. The mixture was washed with aqueous sodium bicarbonate and the organic phase was dried (MgSO4), filtered and concentrated. The residue was purified by column chromatography eluting with gradients of heptane and ethyl acetate to give the title compound (1.28 g, 76%).1H NMR (400 MHz, CDCl3) delta ppm 8.46 (d, 1 H) 7.77 (d, 1 H) 3.74 (m., 2 H) 3.11 - 3.16 (m, 2 H) 1.64 - 1.71 (m, 4 H) 1.55 (m, 2 H) MS (ESI) m/z 259; 261 (M+l). |
|
With thionyl chloride; N,N-dimethyl-formamide; In toluene; for 2h;Reflux; |
A mixture of 3,5-dichloro-pyndme-2-carboxylic acid (5 76 g, 30 mmol) and thionyl chloride (44 mL, 60mmol) in 50 mL of anhydrous toluene and 0 5 mL of anhydrous DMF was refluxed for 2 h The solvent was removed under reduced pressure to give a mobile oil residue which was azeoptoped with toluene (2OmL) The residue was dissolved in 20 mL of anhydrous THF and this solution was added dropwise to a solution of ethyl 3-(dimethylamino)acrylate (47g, 33 mmol) and triethylamine (3 64 g, 36 mmol) in 20 mL of anhydrous THF under nitrogen, and the mixture was heated under reflux for 7 hours The reaction mixture was allowed to cool to room temperature and concentrated under reduced pressure Water (10OmL) and ethyl acetate (10OmL) was added to allow partitioning The organic layer was washed successively with saturated aqueous sodium bicarbonate(x2), water, brine, dried over sodium sulfate and was concentrated under reduced pressure The crude product was purified by ISCO (hexane/EtOAc, 0-40%, 30 mm, 100%, 20 mm) to give 5 9 g (62%) of pure product as yellow oil |
| 110 mg |
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; toluene; at 0 - 20℃; for 1.5h; |
3,5-Dichloropicolinic acid (200 mg, 1.04 mmol) was suspended in dichloromethane (4 mL), the suspension was cooled to 0-5C (ice bath) and oxalyl chloride (363 mg, 250 mu, 2.86 mmol,) as well as a drop of a mixture of dimethylformamide and toluene (1:3, v/v) were added. The mixture was stirred for 1.5 h at room temperature. Then, it was concentrated in vacuo at 40C, the residue was treated with n-heptane (4 mL) and again concentrated and dried in vacuo (40C, 5 mbar) to afford 3,5-dichloropicolinoyl chloride as yellow solid (110 mg). After that, tert-butyl ((4aR,5R,9R)-5-(6-amino-3-fluoropyridin-2-yl)-5,8,8-trimethyl-9-oxido-2,3,4,4a,5,8- hexahydro-[l,4]thiazino[2,l-f] [l,2]thiazin-7-yl)carbamate (Int-17AA, 100 mg, 228 mupiiotaomicron) was dissolved in dichloromethane (4 mL), the solution cooled to 0-5C (ice bath) and N,N- diisopropylethylamine (75.5 mg, 100 mu, 584 muiotaetaomicron) was added, followed by a solution of 3,5- dichloropicolinoyl chloride (vide supra, 60 mg, 285 muiotaetaomicron) in dichloromethane (1.2 mL).The reaction mixture was stirred at 0-5C for 1.5 h. Then, ethanol (100 mu) was added, the mixture was stirred for 45 min at room temperature, poured onto a saturated aqueous solution of sodium hydrogencarbonate (15 mL) and extracted with dichloromethane (1 x 30 mL, 2 x 20 mL). The combined extracts were dried (sodium sulfate) and concentrated in vacuo. The crude was purified by column chromatography (silica gel, 50 g, eluting with ethyl acetate / n-heptane, gradient 60:40 to 80:20) to yield, after drying in vacuo (50C, 5 mbar), the title compound as a light yellow foam (140 mg, 99% yield). HPLC (method LCMS_gradient) tR = 3.2 min. XH NMR (CDC13, 400 MHz): delta 1.48 (s, 9 H), 1.79 (s, 3 H), 1.81-2.02 (m, 3 H), 1.85 (s, 3 H), 1.95 (s, 3 H), 2.40 (dddd, J = 3.8, 12.6, 12.6, 12.6 Hz, 1 H), 3.36-3.43 (m, 1 H), 3.58-3.66 (m, 1 H), 4.08 (dd, J = 3.2, 12.4 Hz, 1 H), 7.57 (dd, .7 = 8.9, 10.7 Hz, 1 H), 7.95 (d, 7 = 2.1 Hz, 1 H), 8.44 (dd, / = 3.1, 9.0 Hz, 1 H), 8.57 (d, J = 1.9 Hz, 1 H), 10.16 (br s, 1 H, exch), 11.23 (br s, 1 H, exch). MS (ES+) m/z 613.3, 615.3 & 617.3 [M+H, 2 CI] . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping