Alternatived Products of [ 80286-58-4 ]
Product Details of [ 80286-58-4 ]
| CAS No. : | 80286-58-4 |
MDL No. : | MFCD00238540 |
| Formula : |
C15H22O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | PLQMEXSCSAIXGB-SAXRGWBVSA-N |
| M.W : |
234.33
|
Pubchem ID : | 10922465 |
| Synonyms : |
Qing Hao acid;Artemisinic acid;Arteannuic acid
|
Chemical Name : | 2-((1R,4R,4aS,8aR)-4,7-Dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalen-1-yl)acrylic acid |
Application In Synthesis of [ 80286-58-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 80286-58-4 ]
- 1
-
 [ 186581-53-3 ]
[ 186581-53-3 ]

-
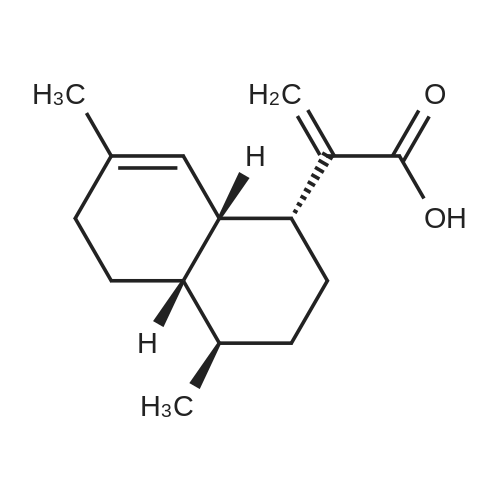 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 129165-35-1 ]
[ 129165-35-1 ]
- 2
-
 [ 186581-53-3 ]
[ 186581-53-3 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 82869-24-7 ]
[ 82869-24-7 ]
Reference:
[1]Journal of Natural Products,2001,vol. 64,p. 1201 - 1205
[2]Tetrahedron Letters,2001,vol. 42,p. 3997 - 4000
[3]Journal of Organic Chemistry,1986,vol. 51,p. 5417 - 5419
[4]Heterocycles,1994,vol. 39,p. 23 - 30
[5]Heterocycles,2000,vol. 53,p. 261 - 264
[6]Journal of Medicinal Chemistry,1990,vol. 33,p. 1516 - 1518
[7]Phytochemistry,1999,vol. 52,p. 843 - 854
[8]Journal of Medicinal Chemistry,2002,vol. 45,p. 4940 - 4944
- 3
-
 [ 186581-53-3 ]
[ 186581-53-3 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 87391-99-9 ]
[ 87391-99-9 ]

-
 [ 85788-54-1 ]
[ 85788-54-1 ]
- 4
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 101020-89-7 ]
[ 101020-89-7 ]
- 5
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
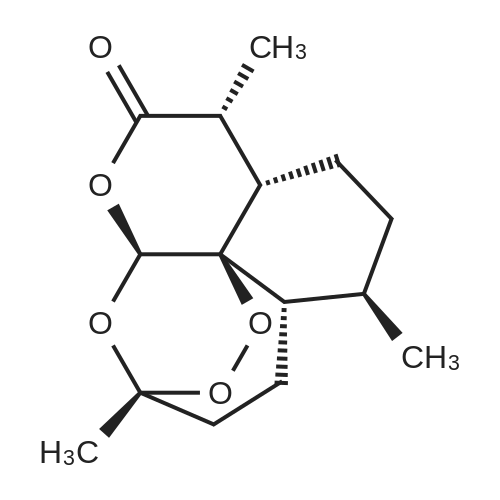 [ 63968-64-9 ]
[ 63968-64-9 ]

-
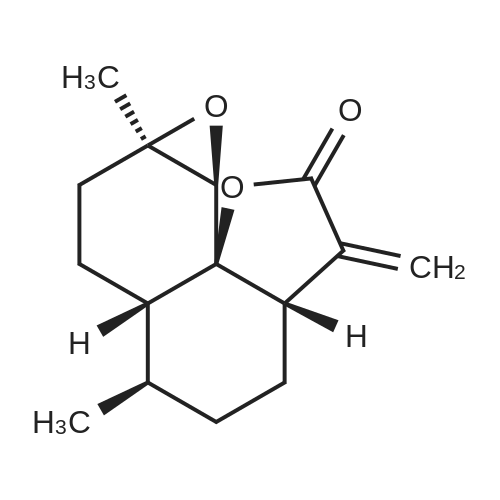 [ 50906-56-4 ]
[ 50906-56-4 ]
- 6
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
arteannuin B
[ No CAS ]
- 7
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
2-((1S,4R,4aS,6S,8aR)-6-Hydroxy-4,7-dimethyl-1,2,3,4,4a,5,6,8a-octahydro-naphthalen-1-yl)-acrylic acid
[ No CAS ]
- 8
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
2-((1S,4R,4aS,6S,8aR)-6-Hydroxy-4,7-dimethyl-1,2,3,4,4a,5,6,8a-octahydro-naphthalen-1-yl)-acrylic acid
[ No CAS ]

-
3-α-hydroxyartemisinic acid
[ No CAS ]
- 9
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 128261-36-9 ]
[ 128261-36-9 ]
- 10
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 128261-36-9 ]
[ 128261-36-9 ]

-
epi-deoxyarteannuin B
[ No CAS ]
- 11
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
epi-deoxyarteannuin B
[ No CAS ]
- 12
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
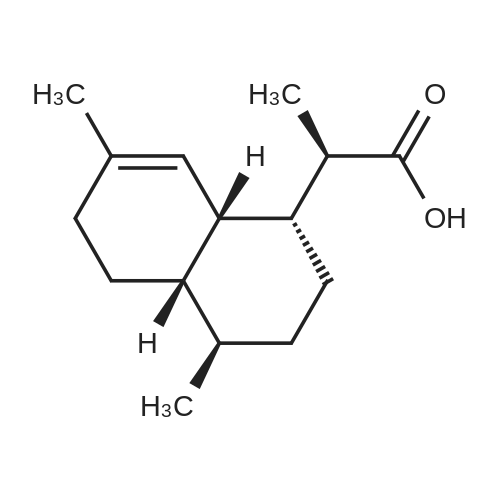 [ 85031-59-0 ]
[ 85031-59-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98% |
With palladium on activated charcoal; hydrogen; In chloroform; at 20℃; under 750.075 Torr; |
Dissolve 100 g (0.43 mol) of <strong>[80286-58-4]artemisinic acid</strong> in 800 mL of chloroform.At room temperature, hydrogen pressure 1 bar, 1.5g palladium carbon as a catalyst, reaction overnight, diatomaceous earth filtration,Wash with chloroform and recover the solvent under reduced pressure.The solid product dihydro<strong>[80286-58-4]artemisinic acid</strong> 99 g (0.42 mol) was obtained in 98% yield. |
| 98.74% |
With dihydrogen peroxide; hydrazine hydrate; In ethanol; at -10℃; for 4h; |
To 1000 g (4.2675 mol) of <strong>[80286-58-4]artemisinic acid</strong> was added 3000 ml of absolute ethanol and 2000 ml (35.02 mol) of 85% hydrazine hydrate, and 1800 ml (17.6294 mol) of 30% hydrogen peroxide was added dropwise at a controlled temperature of -10 C. After 4H, the reaction was completed and 6N was added dropwise Aqueous solution of hydrochloric acid to pH = 1 to obtain 996.6 g (4.2170 mol) of dihydro<strong>[80286-58-4]artemisinic acid</strong> with a yield of 98.74%. |
| 95% |
|
Example 3: Synthesis of dihydro<strong>[80286-58-4]artemisinic acid</strong> la by diimine generation from hydroxylamine and HOSA/NaOH in methanol at -5C to 0C2.34 g (0.01 mol) of <strong>[80286-58-4]artemisinic acid</strong> Ilia was dissolved in 20 mL of MeOH. Then 1 .98 g (0.03 mol) of hydroxylamine (50 % in water) and 5.65 g (0.045 mol) of HOSA (dissolved in 10 mL of water) were added continuously while a pH 9 was held maintained with a 32% aqueous NaOH solution. The temperature was adjusted to between -5C and 0C. After complete addition the reaction mixture was stirred for one additional hour until no pH change was detectable. The complete consumption of <strong>[80286-58-4]artemisinic acid</strong> was confirmed with RP-HPLC analysis. Then the reaction mixture was acidified with dilute aqueous hydrochloride acid to pH 2. The product was extracted with MTBE, dried over magnesium sulfate and the solvent was evaporated to give 2.25 g (95%) of the title compound which crystallized on standing. The diastereomeric ratio in the unpurified product as determined by 1 H- NMR and LC/MS analysis was 98:2 in favour of the desired stereoisomer la. |
| 94% |
With hydrazine hydrate; In isopropyl alcohol; at 15 - 40℃; for 10h; |
15 g (64 mmol) of <strong>[80286-58-4]artemisinic acid</strong> were initially charged in 45 ml of isopropanol. At 15-25 C, 9.81 g (192 mmol) of hydrazine hydrate were added dropwise to the <strong>[80286-58-4]artemisinic acid</strong> solution. The reaction solution were then transferred into a heatable chromatography column with bottom glass frit and, at 40 C, gassed with 5% strength synthetic air (5% O2 + 95% N2) via the bottom glass frit for 10 hours. After complete conversion into dihydro<strong>[80286-58-4]artemisinic acid</strong> (checked by Raman spectroscopy) the reaction solution was discharged into a vessel and the chromatography column was washed twice with 15 ml of isopropanol. The reaction solution (pH 8) was adjusted to pH 3-4 using at least 40 ml of 2N hydrochloric acid. After addition of 75 ml of methylene chloride, the two-phase mixture was stirred vigorously for 10 minutes. Organic and aqueous phase were then separated. The organic phase was extracted once more with 50 ml of water. The resulting methylene chloride solution comprises 14.2 g of dihydro<strong>[80286-58-4]artemisinic acid</strong> in a yield of 94% and a diastereomer ratio of 97:3 (HPLC). |
| 94% |
With hydrazine hydrate; In isopropyl alcohol; at 15 - 40℃; for 10h; |
15 g (64 mmol) of <strong>[80286-58-4]artemisinic acid</strong> were initially charged in 45 ml of isopropanol. At 15- 25C, 9.81 g (192 mmol) of hydrazine hydrate were added dropwise to the <strong>[80286-58-4]artemisinic acid</strong> solution. The reaction solution were then transferred into a heatable chromatography column with bottom glass frit and, at 40 C, gassed with 5% strength synthetic air (5% 02 + 95% N2) via the bottom glass frit for 10 hours. After complete conversion into dihydro<strong>[80286-58-4]artemisinic acid</strong> (checked by Raman spectroscopy) the reaction solution was discharged into a vessel and the chromatography column was washed twice with 15 ml of isopropanol. The reaction solution (pH 8) was adjusted to pH 3-4 using at least 40 ml of 2N hydrochloric acid. After addition of 75 ml of methylene chloride, the two-phase mixture was stirred vigorously for 10 minutes. Organic and aqueous phase were then separated. The organic phase was extracted once more with 50 ml of water. The resulting methylene chloride solution comprises 14.2 g of dihydro<strong>[80286-58-4]artemisinic acid</strong> in a yield of 94% and a diastereomer ratio of 97:3 (HPLC). |
|
With iron(III) chloride; hydrazine hydrate; In ethanol; at 25℃; for 4h; |
5g of <strong>[80286-58-4]artemisinic acid</strong> was dissolved in 25ml of anhydrous ethanol, and then adding 3.2ml mass concentration of 50% hydrazine hydrate and 1 ml aqueous solution containing 30mg of anhydrous ferric chloride, the reaction temperature is adjusted to 25 C, again the reaction solution to 60ml / min of air was fed, the reaction after 4 hours HPLC indicated the reaction has been completed; to the reaction mixture was added 100ml of water, and extracted twice with 50ml butyl ether methyl t, the organic phase was dried over anhydrous sodium dried, filtered, concentrated and the residue of crude dihydro<strong>[80286-58-4]artemisinic acid</strong> 5. 5g, by HPLC showed a purity of 90%. |
|
With guanidinium sulfate; hydrazine hydrate; In water; butan-1-ol; at 100℃; for 7h; |
2.34 g of <strong>[80286-58-4]artemisinic acid</strong> was dissolved in 15 ml of anhydrous n-butanol, and then 1.5 ml of 50% hydrazine hydrate and 1 ml of an aqueous solution of guanidine sulfate containing 50 mg were added. The reaction temperature was adjusted to 100C, and 30% The reaction was completed 3 hours after the incubation reaction; 50 ml of water was added and the mixture was extracted twice with 30 ml of methyl t-butyl ether, and the organic phase was washed with anhydrous sulfuric acid (20 ml) and the organic phase was treated with anhydrous sulfuric acid Sodium dried, filtered and concentrated. The resulting residue was obtained as crude dihydro<strong>[80286-58-4]artemisinic acid</strong> (2.45 g), and the purity was 92.3% by HPLC |

Reference:
[1]Journal of Organic Chemistry,1986,vol. 51,p. 5417 - 5419
[2]Organic Letters,2016,vol. 18,p. 2122 - 2125
[3]Patent: CN107793429,2018,A .Location in patent: Sheet 0022; 0023; 0027; 0031
[4]Patent: CN110590802,2019,A .Location in patent: Paragraph 0074-0075
[5]Patent: WO2011/30223,2011,A2 .Location in patent: Page/Page column 17; 18
[6]Patent: EP2660234,2013,A1 .Location in patent: Paragraph 0039
[7]Patent: WO2013/164367,2013,A1 .Location in patent: Page/Page column 13; 14
[8]Organic Process Research and Development,2014,vol. 18,p. 417 - 422
[9]Patent: CN103755550,2016,B .Location in patent: Paragraph 0018; 0027; 0028
[10]Patent: CN103739476,2016,B .Location in patent: Paragraph 0035; 0036
- 13
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
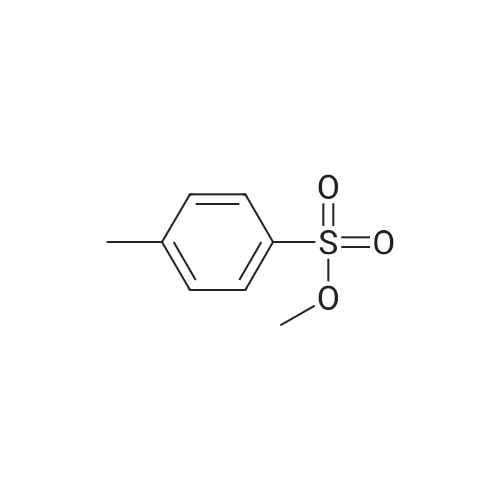 [ 80-48-8 ]
[ 80-48-8 ]

-
 [ 82869-24-7 ]
[ 82869-24-7 ]
- 14
-
 [ 75-77-4 ]
[ 75-77-4 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 195060-78-7 ]
[ 195060-78-7 ]
- 15
-
 [ 13154-24-0 ]
[ 13154-24-0 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 195060-80-1 ]
[ 195060-80-1 ]
- 16
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 18162-48-6 ]
[ 18162-48-6 ]

-
 [ 195060-79-8 ]
[ 195060-79-8 ]
- 17
-
 [ 186581-53-3 ]
[ 186581-53-3 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 150665-64-8 ]
[ 150665-64-8 ]
- 18
-
 [ 13154-24-0 ]
[ 13154-24-0 ]

-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 195060-79-8 ]
[ 195060-79-8 ]
- 19
-
 [ 80286-58-4 ]
[ 80286-58-4 ]

-
 [ 18162-48-6 ]
[ 18162-48-6 ]

-
 [ 195060-80-1 ]
[ 195060-80-1 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












