Alternatived Products of [ 7749-47-5 ]
Product Details of [ 7749-47-5 ]
| CAS No. : | 7749-47-5 |
MDL No. : | MFCD00059768 |
| Formula : |
C6H9N3O
|
Boiling Point : |
No data available |
| Linear Structure Formula : | C4HN2NH2(CH3O)CH3 |
InChI Key : | SNWZXTZIZWBIDQ-UHFFFAOYSA-N |
| M.W : |
139.15
|
Pubchem ID : | 587236 |
| Synonyms : |
|
Application In Synthesis of [ 7749-47-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 7749-47-5 ]
- 1
-
 [ 5600-21-5 ]
[ 5600-21-5 ]

-
 [ 124-41-4 ]
[ 124-41-4 ]

-
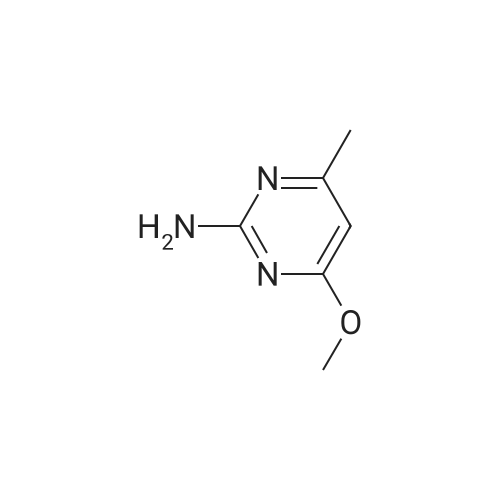 [ 7749-47-5 ]
[ 7749-47-5 ]
- 2
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
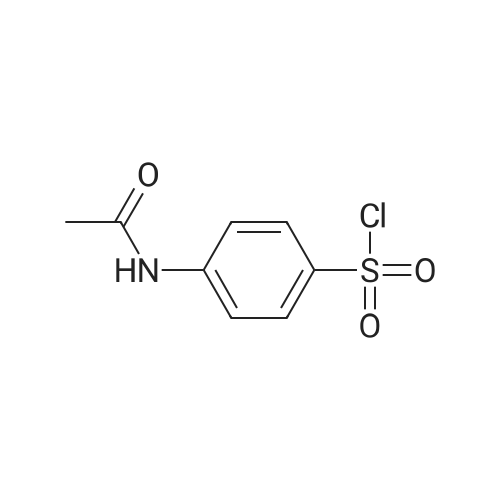 [ 121-60-8 ]
[ 121-60-8 ]

-
 [ 36082-36-7 ]
[ 36082-36-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 20% |
With pyridine; at 0 - 60℃; for 17.0h; |
Step 1: Synthesis of N-{4-[(4-methoxy-6-methylpyrimidin-2-yl)sulfamoyl] phenyl}acetamide 19.1 [00351] 4-(Acetylamino)benzenesulfonyl chloride (2.00 g, 8.56 mmol) was added at 0C to a stirred solution of <strong>[7749-47-5]4-methoxy-6-methylpyrimidin-2-amine</strong> (1.19 g, 8.56 mmol) in pyridine (dry, 20ml). The reaction mixture was allowed to reach rt and stirred for 15h then heated to 60C for 2h. The solvent was removed in vacuo by azeotroping with toluene (2 x 25ml), followed by n- heptane (2 x 25ml) to afford a brown oil which was purified by flash column chromatography (MeOH/DCM 0/100 to 5/95) to obtain 0.60g (20%) of N-{4-[(4-methoxy-6-methylpyrimidin-2- yl)sulfamoyl]phenyl}acetamide 19.1 a colourless solid. |
|
With pyridine; at 60℃; for 12.0h; |
Step 1 - N-[4-[(4-methoxy-6-methyl-pyrimidin-2-yl)sulfamoyl]phenyl]acetamide (0431) [00295] To a suspension of 4-methoxy-6-methyl-pyrimidin-2-amine (4.00 g, 28.8 mmol) in pyridine (40 mL) was added 4-acetamidobenzenesulfonyl chloride (7.05 g, 30.2 mmol, CAS121-60-8). The reaction mixture was stirred at 60 C for 12 hr. On completion, the reaction mixture was concentrated and the residue was diluted with water, the solid was filtered, washed with dichloromethane (30 mL), and dried under vacuum to give the title compound. LCMS: (ES- ) m/z (M-H)- = 335.1, tR = 1.011. |
- 3
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
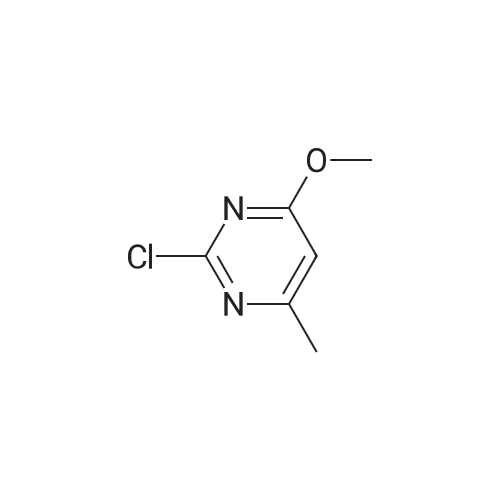 [ 22536-64-7 ]
[ 22536-64-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 8% |
With hydrogenchloride; sodium nitrite; In water; at 0 - 20℃; for 1.5h; |
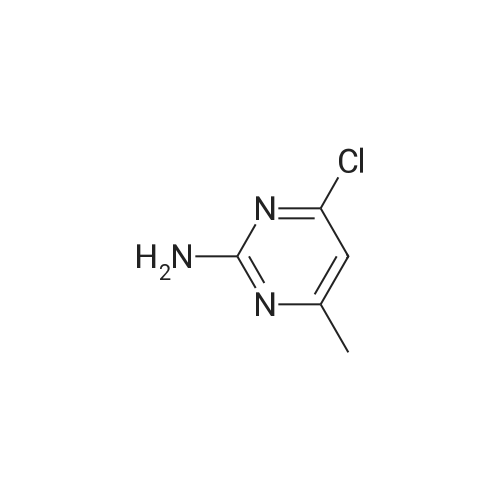
To a solution 2-amino-4-methoxy-6-methylpyrimidine (5 g, 35.97 mmol) in aqueous concentrated HCl (50 ml) was added NaNO2 (2.97 g, 43.16 mmol) in water(5 ml) slowly drop by drop over a period 30 min at 0oC and then the reaction mixture was stirred at room temperature for 1 hour. Then, the reaction mixture was quenched with 10 N sodium hydroxide solution then insoluble material was, filtered and filtrate was partitioned between brine and AcOEt and the aqueous layer was extracted with AcOEt. The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give a brown oil. The crude compound which upon purification by flash chromatography using (silica-gel: 100-200 mesh, AcOEt -petroleum ether; 10:90→ 15:85) gave 600 mg (yield 8%) of a white solid corresponding to 2-chloro-4-methoxy-6- methylpyrimidine. |
|
With sodium nitrite; In hydrogenchloride; water; |
EXAMPLE 6 2-Chloro-4-methyl-6-methoxypyrimidine A solution of 25.4 g of 2-amino-4-methyl-6-methoxypyrimidine in 150 ml concentrated hydrochloric acid was cooled to ca. 0 C. and treated with a solution of 25.3 g of sodium nitrite in 50 ml water, added over a period of 30-40 minutes. The thick orange reaction mixture was stirred at room temperature for 4-5 hours and then adjusted to pH 10 by addition of 12.5N sodium hydroxide solution. The precipitate was filtered and extracted thoroughly with approximately 600 ml of hot ether. The organic layer was dried over magnesium sulfate and concentrated in vacuo to give 7.2 g of 2-chloro-4-methyl-6-methoxypyrimidine as a white, crystalline solid, m.p. 36-38 C. NMR(CDCl3): δ 6.5 (1H, s, heterocyclic H); 4.0 (3H, s, --OCH3); 2.4 (3H, s, --CH3). |
Reference:
[1]Patent: WO2017/191599,2017,A1 .Location in patent: Page/Page column 176; 177
[2]Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan,1953,vol. 73,p. 598,600
Chem.Abstr.,1954,p. 9362
[3]Patent: US4496392,1985,A
- 4
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
3-Isocyanatosulfonyl-1-methyl-1H-indole-2-carboxylic acid methyl ester
[ No CAS ]

-
3-[[(4-methoxy-6-methylpyrimidin-2-yl)aminocarbonyl]aminosulfonyl]-1-methyl-1H-indole-2-carboxylic acid, methyl ester
[ No CAS ]
- 5
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
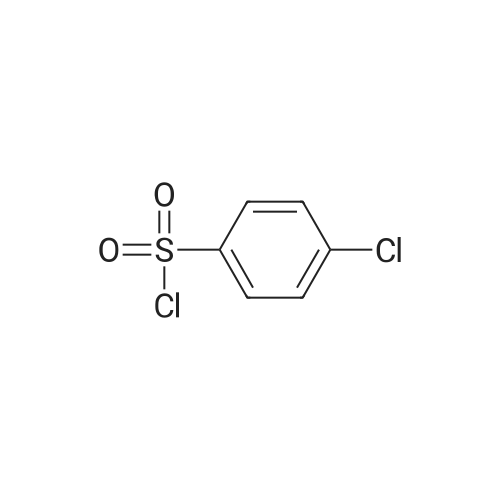 [ 98-60-2 ]
[ 98-60-2 ]

-
 [ 353302-84-8 ]
[ 353302-84-8 ]
- 6
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
 [ 98-59-9 ]
[ 98-59-9 ]

-
 [ 330946-56-0 ]
[ 330946-56-0 ]
- 7
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
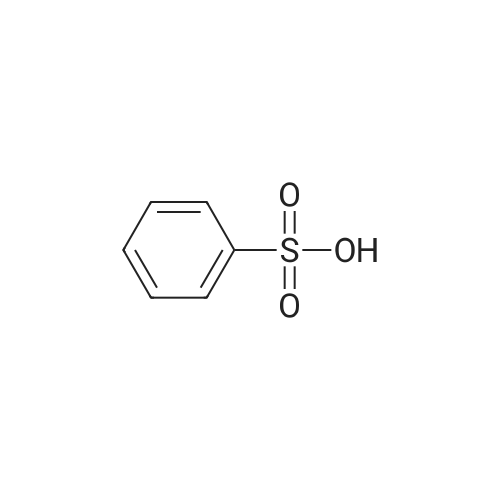 [ 98-11-3 ]
[ 98-11-3 ]

-
 [ 312597-84-5 ]
[ 312597-84-5 ]
- 8
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
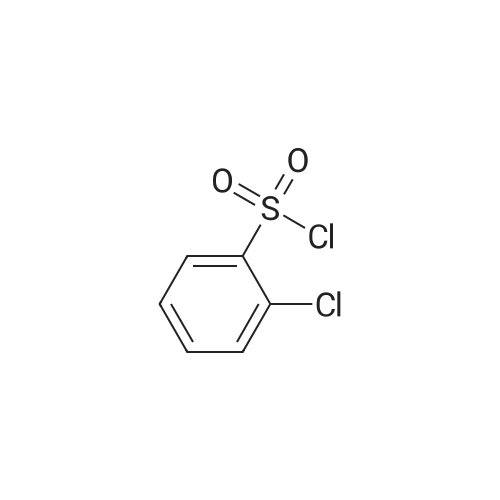 [ 2905-23-9 ]
[ 2905-23-9 ]

-
 [ 130240-03-8 ]
[ 130240-03-8 ]
- 9
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
 [ 140160-21-0 ]
[ 140160-21-0 ]

-
1-(4-methoxy-6-methyl-pyrimidin-2-yl)-3-[5-(4-nitro-phenyl)-furan-2-carbonyl]-thiourea
[ No CAS ]
- 10
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
 [ 304437-60-3 ]
[ 304437-60-3 ]

-
1-[5-(2-chloro-phenyl)-furan-2-carbonyl]-3-(4-methoxy-6-methyl-pyrimidin-2-yl)-thiourea
[ No CAS ]
- 11
-
 [ 936020-75-6 ]
[ 936020-75-6 ]

-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
C12H15N5O2S2
[ No CAS ]
- 12
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
5-Methoxy-2,7-dimethyl-imidazo[1,2-a]pyrimidine-3-carboxylic acid
[ No CAS ]
- 13
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
5-methoxy-7-methylimidazo<1,2-a>pyrimidine-2-carboxylic acid
[ No CAS ]
- 14
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
5-methoxy-7-methylimidazo<1,2-a>pyrimidine-2-carboxamide
[ No CAS ]
- 15
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
4-amino-N-(4-methoxy-6-methylpyrimidin-2-yl)benzene-1-sulfonamide
[ No CAS ]
Reference:
[1]Recueil des Travaux Chimiques des Pays-Bas,1942,vol. 61,p. 291,297
[2]Recueil des Travaux Chimiques des Pays-Bas,1942,vol. 61,p. 291,297
[3]Patent: WO2016/40449,2016,A1
- 16
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
ethyl (N-methylsulfonyl-N-methylamino)sulfonylcarbamate
[ No CAS ]

-
1-[(N-methylsulfonyl-N-methylamino)-sulfonyl]-3-(4-methoxy-6-methyl-2-pyrimidinyl)-urea
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 96.4% |
In chlorobenzene; |
EXAMPLE 2 1-[(N-Methylsulfonyl-N-methylamino)sulfonyl]-3-(4-methoxy-6-methyl-2-pyrimidyl)urea 52.0 g of ethyl (N-methylsulfonyl-N-methylamino)sulfonylcarbamate are dissolved in 500 ml of chlorobenzene, 27.8 g of 2-amino-4-methoxy-6-methylpyrimidine are added at room temperature, and the mixture is heated at 50 C. for 5 hours. After cooling to 0 C., the precipitate is filtered off. After washing with 100 ml of chlorobenzene, 68.8 g of the desired product of a purity of 98.9% are obtained; this corresponds to a yield of 96.4% of theory. The melting point of the product is 118-120 C. |
- 17
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
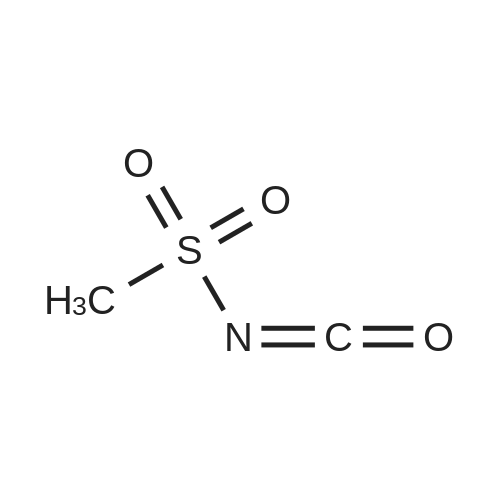 [ 3611-92-5 ]
[ 3611-92-5 ]

-
1-[(N-methylsulfonyl-N-methylamino)-sulfonyl]-3-(4-methoxy-6-methyl-2-pyrimidinyl)-urea
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In dichloromethane; |
EXAMPLE 19 1-[(N-methylsulfonyl-N-methylamino)-sulfonyl]-3-(4-methoxy-6-methyl-2-pyrimidinyl)-urea 13.9 g (0.1 mol) of 2-amino-4-methoxy-6-methylpyrimidine are suspended in 150 ml of methylene dichloride, and 21.4 g (0.1 mol) of N-methylsulfonyl isocyanate in 50 ml of methylene dichloride are added at 0 C. in the course of one hour. Stirring is continued for 15 hours at room temperature and the product is then precipitated with n-hexane. 20.4 g (58% of theory) of 1-[(N-methylsulfonyl-N-methylamino)-sulfonyl]-3-(4-methoxy-6-methyl-2-pyrimidinyl)-urea of melting point 118-120 C. are obtained. |
- 18
-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
 [ 114332-63-7 ]
[ 114332-63-7 ]

-
 [ 60-29-7 ]
[ 60-29-7 ]

-
 [ 4223-09-0 ]
[ 4223-09-0 ]

-
 [ 102410-14-0 ]
[ 102410-14-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In acetonitrile; |
EXAMPLE 8 N-[(4-Methyl-6-methoxypyrimidin-2-yl)aminocarbonyl]-2-[5-(methylthio)-1,3,4-oxadiazol-2-yl]benzenemethanesulfonamide To a suspension of 0.33 g of 2-amino-4-methyl-6-methoxypyrimidine in 10 ml of acetonitrile was added 1 g of crude sulfonyl isocyanate prepared in Example 7. The suspension was stirred at room temperature overnight, then filtered. The residue was washed with 25 ml of ethyl ether and suction dried to give 0.7 g of the subject compound; m.p. 194-196 C. NMR(CDCl3, DMSO): ppm δ2.3 (s, 3H, CH3) 2.8 (s, 3H, SCH3); 3.8 (s, 3H, OCH3); 5.6 (s, 2H, CH2); 6.3 (s, 1H, pyrimidin); 7.4-7.9 (m, 4H, arom.). IR (nujol): 1710 cm-1 (C=O). |
- 19
-
ammonium thiocyanate
[ No CAS ]

-
 [ 7749-47-5 ]
[ 7749-47-5 ]

-
 [ 98-88-4 ]
[ 98-88-4 ]

-
 [ 93744-71-9 ]
[ 93744-71-9 ]

-
N-[[(4-methoxy-6-methylpyrimidin-2-yl)aminothiocarbonyl]amino]benzamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In acetonitrile; |
EXAMPLE 3 N-[(4-Methoxy-6-methylpyrimidin-2-yl)aminothioxomethyl]benzamide To a hot solution of 14 g of ammonium thiocyanate in 300 ml of acetonitrile was added 24 ml of benzoyl chloride. The mixture was heated on the steam bath for 30 minutes and filtered. The filtrate was heated with 21 g of 4-methoxy-6-methylpyrimidine-2-amine for 30 minutes and cooled. The product was collected by filtration and washed with a little acetonitrile to provide 28 g of N-[[(4-methoxy-6-methylpyrimidin-2-yl)aminothiocarbonyl]amino]benzamide, m.p. 193-195. IR (Nujol) 3290, 1720 cm-1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping