| 97% |
With C76H124N6Nd2O6Si4; caesium carbonate; In dimethyl sulfoxide; at 40℃; under 760.051 Torr; for 24h;Inert atmosphere; |
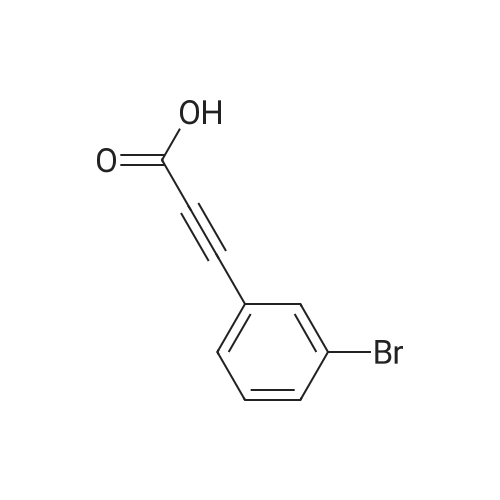
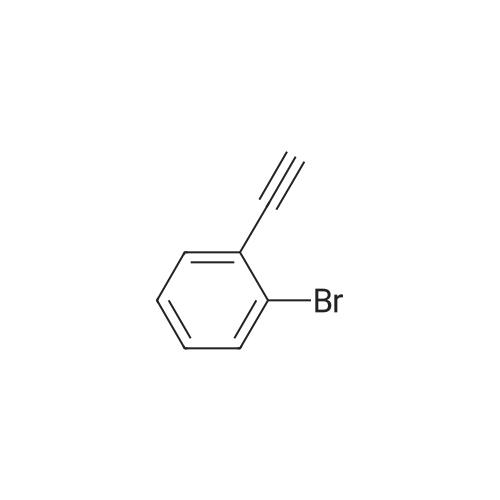
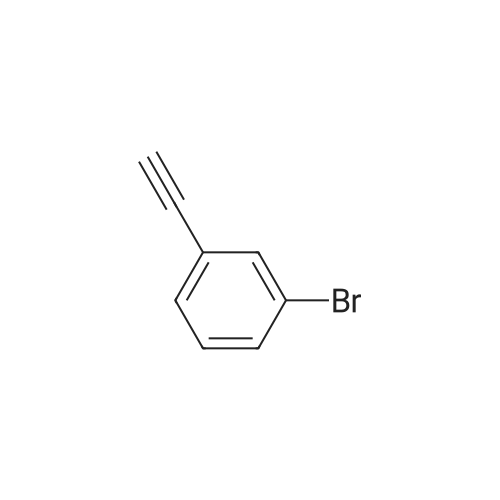
Under anhydrous anaerobic, argon protection, 0.0600 g (3.71 x 10-5 mol) of {LNd [N (SiMe3) 2] · THF} 2 was added to the reaction flask. Then 0.6033 g (1.85 x 10-3 moles) of cesium carbonate was added, under the protection of carbon dioxide bags, 2 ml of dimethyl sulfoxide was added. 0.112 ml (9.28 x 10-4 mol) of 3-bromophenylacetylene was added. Then 3 ml of dimethyl sulfoxide was added. The reaction was stirred in a constant temperature bath at 40 C. After 24 hours, 10 mL of water was added to the quench the reaction, and then filtered. The clear solution was placed in a separatory funnel, a certain amount of hydrochloric acid solution was added to acidify, extracted four times with ether, the extract was washed twice with saturated brine, separated, solvent was spin-dried, and then the residual solvent was removed by pumping to obtain the product. The calculated yield was 97%. |
| 90% |
With caesium carbonate; In dimethyl sulfoxide; at 60℃; under 760.051 Torr; for 24h; |
Add 4 ml of dimethyl sulfoxide, 1 mmol of 3-bromophenylacetylene, and 2 mmol of cesium carbonate into the reaction tube,The reaction tube was pumped and ventilated 3 times, and filled with CO2. After the CO2 was filled, the gas pressure of the reaction tube was 1 atm. The reaction tube was stirred for 24 hours under the conditions of carbon dioxide atmosphere and 60C. The stirring rate is 800 rpm, stop stirring, and cool to room temperature.Add water to the reaction liquid, extract 4 times with ethyl acetate, separate the layers, take the water layer, acidify the water layer with 2 moles of hydrochloric acid per liter to pH=1, then extract with ethyl acetate, take the organic layer, and wash the organic layer with saturated brine It was dried over magnesium sulfate, the filtrate was filtered, and concentrated under reduced pressure to obtain the target product with a yield of 90%. |
| 78% |
With sodium acetate; tetraoctyl ammonium bromide; copper(I) bromide; In acetonitrile; at 25℃; under 11251.1 Torr; for 16h;Inert atmosphere; |
Cuprous bromide (28 mg, 0.2 mmol), sodium acetate (272 mg, 2 mmol), tetra-n-octylammonium bromide (1093.6 mg, 2 mmol), m -bromophenylacetic acid (181 mg, ), Followed by addition to a 25mL reactor, evacuated nitrogen three times, under nitrogen was added refined acetonitrile (4.0mL), charged with CO2 (1.5MPa). The reaction kettle was closed and placed in a 25 C oil bath for 16 h. After the reaction was over, the valve on the kettle was slowly opened to release the remaining gas. The reaction mixture in the autoclave was transferred to a single-neck flask for concentration and diluted with 5 mL of deionized water , And then extracted with n-hexane. The aqueous layer was acidified to pH = 1 at low temperature by addition of 1 M hydrochloric acid and extracted with ether. The organic phase was collected, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and the solvent removed in vacuo to afford M - Bromophenolic acid, yield 78%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping