|
With hydrogenchloride; triphenylphosphine; In tetrahydrofuran; chloroform; di-isopropyl ether; toluene; |
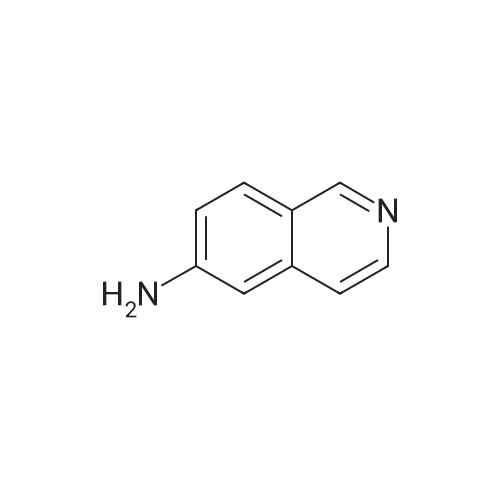
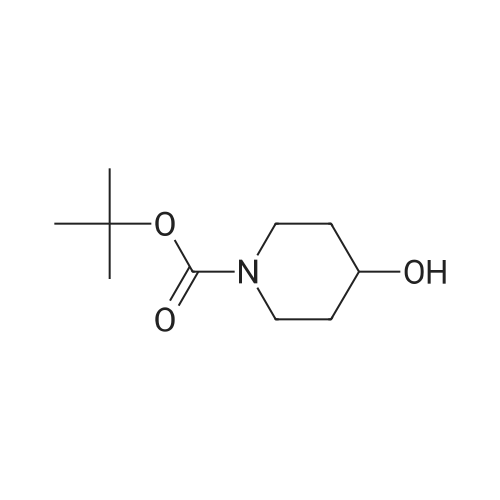
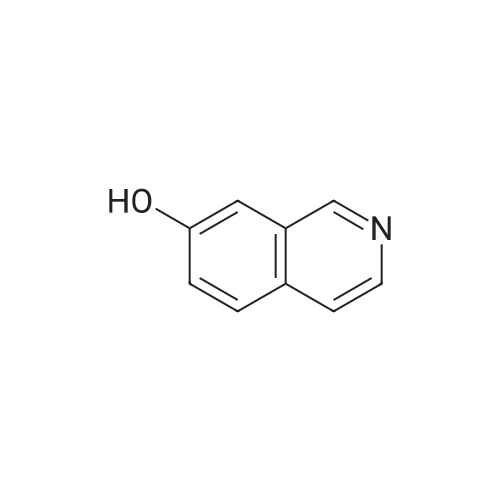
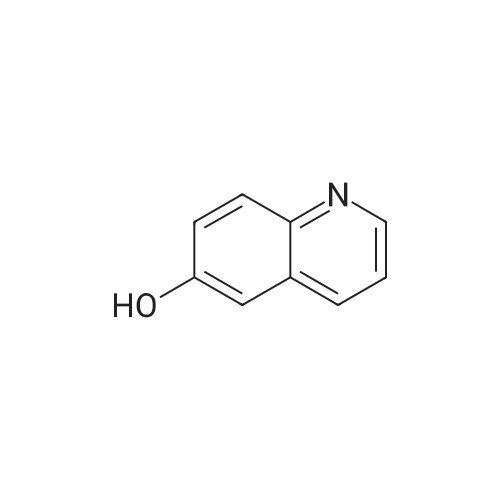
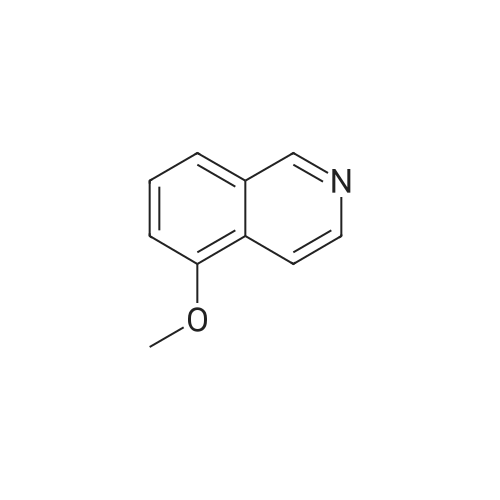
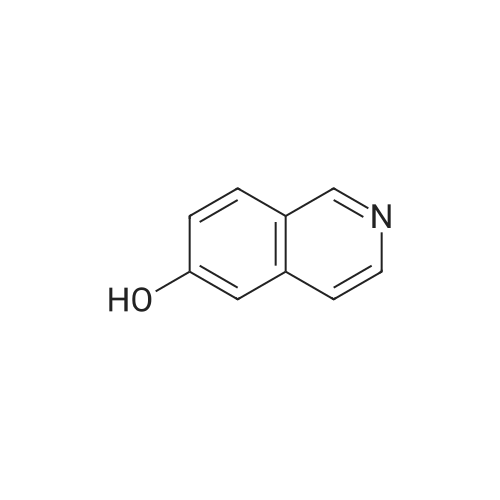
EXAMPLE 34 6-[2-[(3,5-di-tert-butyl-4-hydroxyphenyl)-4-oxazolyl ]ethoxy]isoquinoline hydrochloride monohydrate Title compound was prepared from compound of Example 1C (19.1 mmole, 6.07 g), triphenylphosphine (21.1 mmole, 5.52 g) and <strong>[7651-82-3]6-hydroxyisoquinoline</strong> (21.lmmole, 3.07 g) in tetrahydrofuran (43 ml) at -10° C. (ice/acetone bath) was added diethylazodicarboxylate (21.1 mmole, 3.67 g) over an eleven minute period. After the addition was complete, the reaction was stirred at room temperature. At approximately 3.8 hours the reaction was concentrated in vacuo to an oil. The oil was taken up into chloroform then chromatographed. Material was eluted with 70-85percent ethyl acetate/hexane gradient over a thirty minute period. Fractions containing desired product were combined, reduced in volume and chromatographed. Material was eluted with 0-15percent methanol/toluene gradient over a thirty minute period. Fractions containing desired product were combined and concentrated in vacuo to a solid. The solid was treated with chloroform (100 ml), hydrogen chloride gas was passed through the solution which was then concentrated in vacuo to a yellow foam. The foam was triturated in diisopropyl ether (100 ml) then filtered. Insolubles were treated with toluene (100 ml), heated until boiling, filtered hot, and washed with toluene (50 ml). These insolubles were crystallized from methylene chloride. Crystals were treated with chloroform (60 ml), and then with hydrogen chloride gas and concentrated in vacuo to a foam. Material was triturated in toluene (100 ml) and filtered and the insolubles were collected by filtration to afford 1.38 g of product. Mass Spectrum (ion spray):m/z 444 (M-HCl). 1H NMR (DMSOd6): delta9.71 (s, 1 H), 8.56 (d, 1 H), 8.44 (d, 1 H), 8.28 (d, 1 H), 7.99 (s, 1 H), 7.84 (d, 1 H), 7.73 (s, 2 H), 7.64 (dd, 1 H), 7.56 (bs, 1 H), 4.56 (t, 2 H), 3.13 (t, 2 H), 1.41 (s, 18 H). |
|
With hydrogenchloride; triphenylphosphine; In tetrahydrofuran; chloroform; di-isopropyl ether; toluene; |
Example 34 6-[2-[(3,5-di-tert-butyl-4-hydroxyphenyl)-4-oxazolyl]ethoxy]isoquinoline hydrochloride monohydrate Title compound was prepared from compound of Example 1C (19.1 mmole, 6.07 g), triphenylphosphine (21.1 mmole, 5.52 g) and <strong>[7651-82-3]6-hydroxyisoquinoline</strong> (21.1 mmole, 3.07 g) in tetrahydrofuran (43 ml) at -10° C. (ice/acetone bath) was added diethylazodicarboxylate (21.1 mmole, 3.67 g) over an eleven minute period. After the addition was complete, the reaction was stirred at room temperature. At approximately 3.8 hours the reaction was concentrated in vacuo to an oil. The oil was taken up into chloroform then chromatographed. Material was eluted with 70-85percent ethyl acetate/hexane gradient over a thirty minute period. Fractions containing desired product were combined, reduced in volume and chromatographed. Material was eluted with 0-15percent methanol/toluene gradient over a thirty minute period. Fractions containing desired product were combined and concentrated in vacuo to a solid. The solid was treated with chloroform (100 ml), hydrogen chloride gas was passed through the solution which was then concentrated in vacuo to a yellow foam. The foam was triturated in diisopropyl ether (100 ml) then filtered. Insolubles were treated with toluene (100 ml), heated until boiling, filtered hot, and washed with toluene (50 ml). These insolubles were crystallized from methylene chloride. Crystals were treated with chloroform (60 ml), and then with hydrogen chloride gas and concentrated in vacuo to a foam. Material was triturated in toluene (100 ml) and filtered and the insolubles were collected by filtration to afford 1.38 g of product. Mass Spectrum (ion spray): m/z 444 (M-HCl). 1 H NMR (DMSOd6): delta9.71 (s, 1H), 8.56 (d, 1H), 8.44 (d, 1H), 8.28 (d, 1H), 7.99 (s, 1H), 7.84 (d, 1H), 7.73 (s, 2H), 7.64 (dd, 1H), 7.56 (bs, 1H), 4.56 (t, 2H), 3.13 (t, 2H), 1.41 (s, 18H). Elemental analysis for C28 H33 ClN2 O3.1.0 H2 O: Calculated: C, 67.38; H, 7.07; N, 5.61. Found: C, 67.60; H, 6.87; N, 5.35. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping