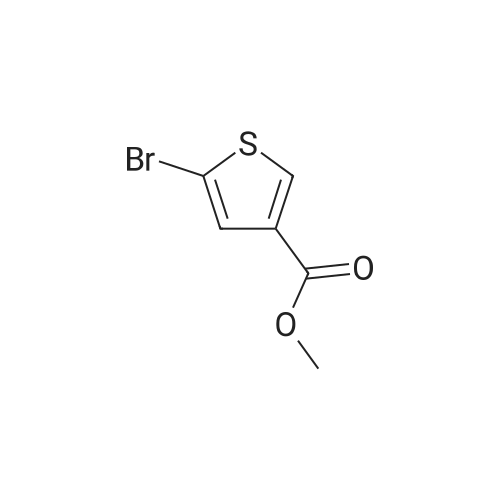
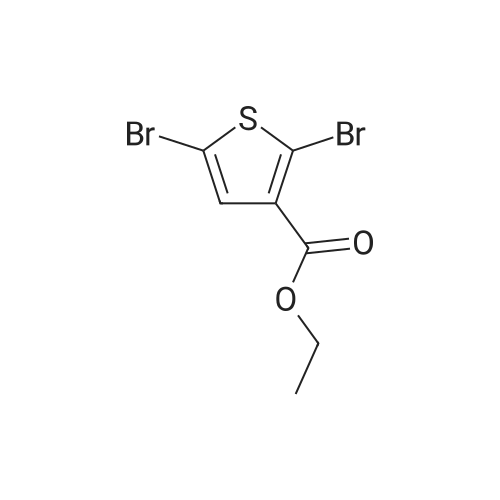
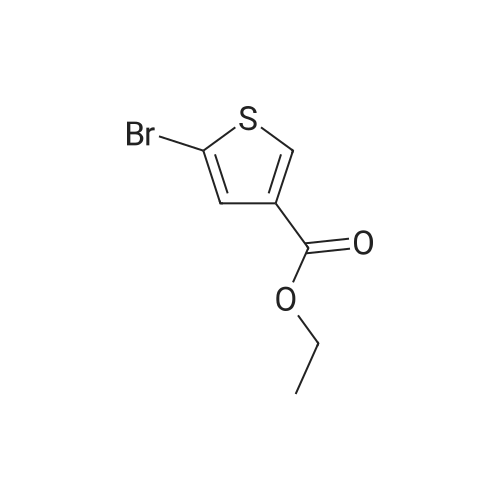
| 50% |
With sodium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 120℃; for 0.166667h;Microwave; |
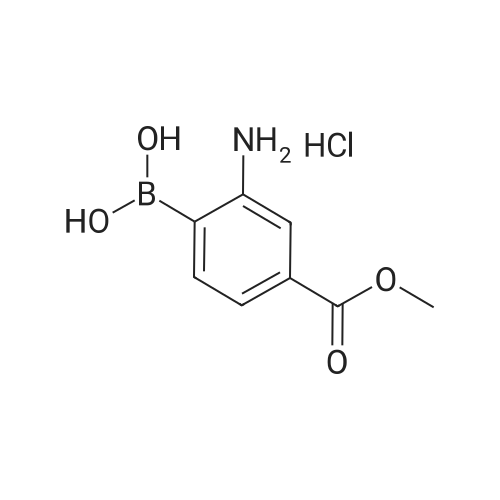
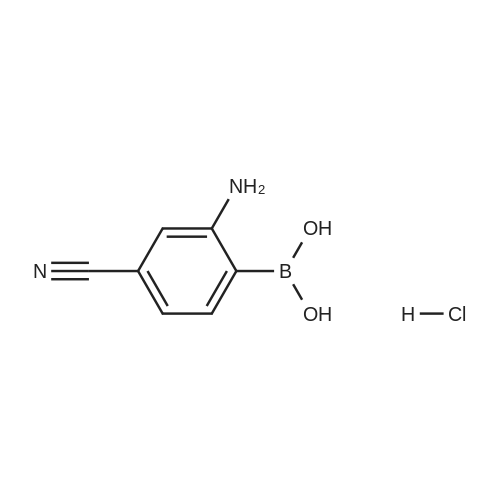
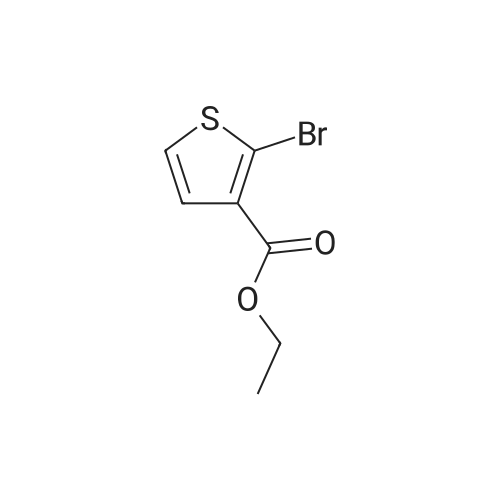
In a microwave vessel, methyl 2-bromo-3-thiophene carboxylate (1.0 eq, 260 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.1 eq, 300 mg, 1.30 mmol), sodium acetate (3.0 eq, 292 mg, 3.56 mmol) and PdCl2(dppf) (0.05 eq, 31 mg, 0.059 mmol) were mixed together in anhydrous DMF (2 ml). The mixture was heated in a microwave oven at 1200C for 10 mn. Water was added and the solid filtered and dried. The material was suspended in CH2Cl2 , filtered and dried to afford methyl 4-oxo-4,5-dihydrothieno[3,2-c]quinoline-7-carboxylate as a yellow solid (152 mg, 50percent yield). LCMS (ES): 95percent pure, m/z 260 [M+l]+ ; <n="104"/>1H NMR (CDCl3, 400 MHz) delta 3. 99 (s, 3H), 7.54 (d, / = 5.2, IH), 7.79 (d, / = 4.8, IH), 7.86 (d, J = 8.4, IH), 7.91 (dd, J = 8.4, / = 1.6, IH), 8.03 (d, J = 1.2, IH) ppm. |
| 50% |
With sodium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 120℃; for 0.166667h;Microwave irradiation; |
Process 11 In a microwave vessel, methyl 2-bromo-3-thiophene carboxylate (1.0 eq, 260 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.1 eq, 300 mg, 1.30 mmol), sodium acetate (3.0 eq, 292 mg, 3.56 mmol) and PdCl2(dppf) (0.05 eq, 31 mg, 0.059 mmol) were mixed together in anhydrous DMF (2 ml). The mixture was heated in a microwave oven at 120° C. for 10 nm. Water was added and the solid filtered and dried. The material was suspended in CH2Cl2, filtered and dried to afford methyl 4-oxo-4,5-dihydrothieno[3,2-c]quinoline-7-carboxylate as a yellow solid (152 mg, 50percent yield). LCMS (ES): 95percent pure, m/z 260 [M+1]+; 1H NMR (CDCl3, 400 MHz) delta 3.99 (s, 3H), 7.54 (d, J=5.2, 1H), 7.79 (d, J=4.8, 1H), 7.86 (d, J=8.4, 1H), 7.91 (dd, J=8.4, J=1.6, 1H), 8.03 (d, J=1.2, 1H) ppm. |
| 50% |
With sodium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 120℃; for 0.166667h;Microwave irradiation; |
In a microwave vessel, methyl 2-bromo-3-thiophene carboxylate (1.0 eq, 260 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.1 eq, 300 mg, 1.30 mmol), sodium acetate (3.0 eq, 292 mg, 3.56 mmol) and PdCl2(dppf) (0.05 eq, 31 mg, 0.059 mmol) were mixed together in anhydrous DMF (2 ml). The mixture was heated in a microwave oven at 120° C. for 10 nm. Water was added and the solid filtered and dried. The material was suspended in CH2Cl2, filtered and dried to afford methyl 4-oxo-4,5-dihydrothieno[3,2-c]quinoline-7-carboxylate as a yellow solid (152 mg, 50percent yield). LCMS (ES): 95percent pure, m/z 260 [M+1]+; 1H NMR (CDCl3, 400 MHz) delta 3.99 (s, 3H), 7.54 (d, J=5.2, 1H), 7.79 (d, J=4.8, 1H), 7.86 (d, J=8.4, 1H), 7.91 (dd, J=8.4, J=1.6, 1H), 8.03 (d, J=1.2, 1H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping