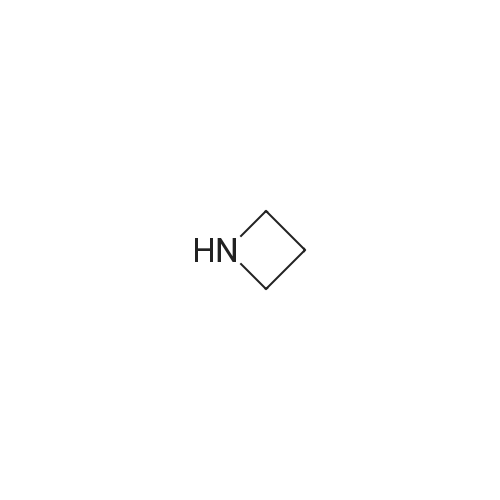
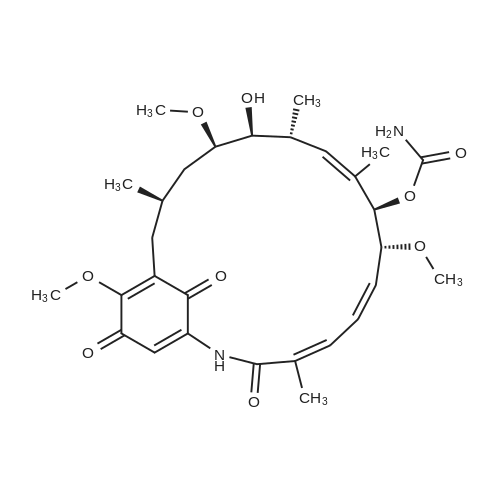
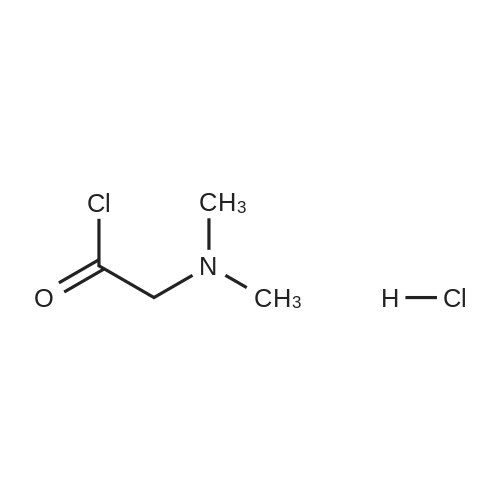
| 99% |
In chloroform; at 20℃; for 18h; |
(+) -Geldanamycin (5.1 mg, 9. 0 mol) was stirred with allylamine (10.0 ul, 0.13 mmol) in chloroform (1.5 ml) at room temperature. Upon the complete conversion of GA shown by thin layer chromatography (18 hours), the mixture was washed with brine, dried over anhydrous sodium sulfate, and concentrated. Separation by flash column chromatography on silica gel (hexane/ethyl acetate) gave the product as a purple solid (5.3 mg, 99%). IR (KBr) (can~') 3464,3333, 2958,2929, 2825,1728, 1691,1652, 1571,1485, 1372,1323, 1189,1101, 1057; UV (95% EtOH) (nm) 332 (s = 2. 0 x 104) ;'H NMR (CDC13, 500 MHz) 8 9. 14 (s, 1H), 7.28 (s, 1H), 6.93 (bd, J= 11.5 Hz, 1H), 6.56 (bdd, J= 11. 5,11. 0 Hz, 1H), 6.38 (bt, J= 6. 0 Hz, 1H), 5.94-5. 81 (m, 3H), 5.30-5. 24 (m, 2H), 5.17 (s, 1H), 4.82 (bs, 2H), 4.29 (bd, J= 10.0 Hz, 1H), 4.21 (bs, 1H), 4.18-4. 08 (m, 2H), 3.55 (ddd, J= 9.0, 6.5, 2.0 Hz, 1H), 3.43 (ddd, J= 9.0, 3.0, 3.0 Hz, 1H), 3. 34 (s, 3H), 3.25 (s, 3H), 2.72 (dqd, J= 9.5, 7.0, 2.0 Hz, 1H), 2.63 (d, J= 14.0 Hz, 1H), 2.34 (dd, J= 14. 0,11. 0 Hz, 1H), 2.00 (bs, 3H), 1.78 (d, J= 1.0 Hz, 3H), 1.78-1. 74 (m, 2H), 1.74-1. 67 (m, 1H), 0.99-0. 95 (m, 6H) ; 3C NMR (CDC13, 125 MHz, assignment of protonated carbons aided by DEPT) 8 183.8 (18-C), 180.9 (21-C), 168.4 (1-C), 156.0 (7- OzCNHz), 144.6 (17-C), 141.2 (20-C), 135.8 (5-C), 134.9 (2-C), 133.7 (9-C), 132.7 (8-C), 132.5 (3'-C), 126.9 (4-C), 126.5 (3-C), 118.5 (3'-C), 108.8 (19-C), 108.7 (16-C), 81.6 (7-C), 81.4 (12-C), 81.2 (6-C), 72.6 (11-C), 57.1 (6-or 12-OCH3), 56.7 (6-or 12-OCH3), 47.8 (1'-C), 35.0 (13-C), 34.3 (15-C), 32.3 (10-C), 28.4 (14-C), 22.9 (14-CH3), 12.8 (8-CH3), 12.6 (2-CH3), 12.3 (10-CH3); HRMS (FAB) found 586.3120 [M+H] +, calcd. 586.3129 for C31H44N30g. |
| 95% |
In dichloromethane; at 23℃; |
17-AAG was synthesized in the lab from geldanamycin (GA) (LC Laboratories, Woburn, MA). Briefly, 100 mg of GA (0.2 mmol) was dissolved in 2 mL of dry CH2Cl2. Next, 5 equivalents of allylamine (57.1 g/mol, d = 0.763 g/mL) was added dropwise to the flask. The reaction was stirred at room temperature (RT; ?23 C) under low light until complete by TLC analysis (approx. 2 days) (95:5 CHCl3:MeOH, Rf 0.21), precipitated with hexane (3x), centrifuged at 2000 g's for 15 minutes, and evaporated to dryness. Yield: 95 mg, 95%; MS m/z 584 (M-); 1H NMR (CDCl3) delta 0.99 (m, 6H, 10-Me, 14-Me), 1.25 (t, 1H, H-13), 1.60-1.85 (br m, 6H, H-13, H-14, 8-Me), 2.05 (s, 3H, 2-Me), 2.46 (br m, 2H, H-15), 2.83-2.90 (br m, 3H, H-10), 3.27 (s, 3H, OMe), 3.36 (s, 3H, OMe), 3.40 (t, 1H, H-12), 3.58-3.68 (br m, 2H, H-11, H-23), 4.31 (d, 1H, H-7), 5.10 (br s, 1H), 5.21-5.55 (br m, 3H, H-9, H-24), 5.86-5.99 (br t, 2H, H-5, H-23), 6.59 (t, 1H, H-4), 6.94 (d, 1H, H-3), 7.28 (br s, 1H, H-19). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













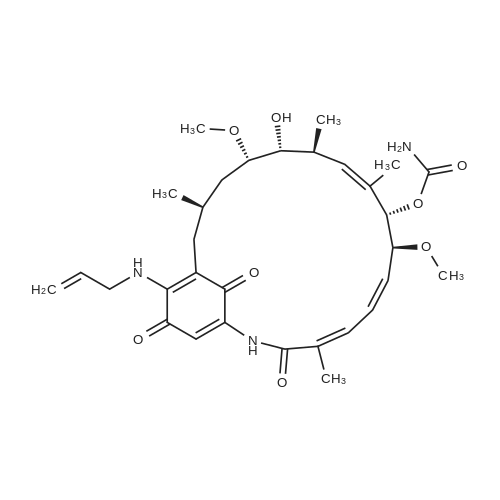

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping