| 84% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; for 18h;Heating / reflux; |
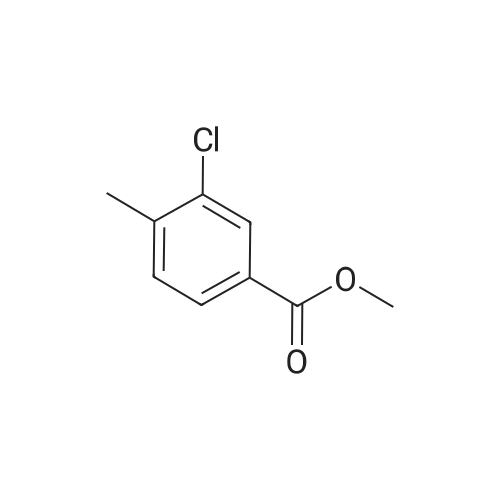
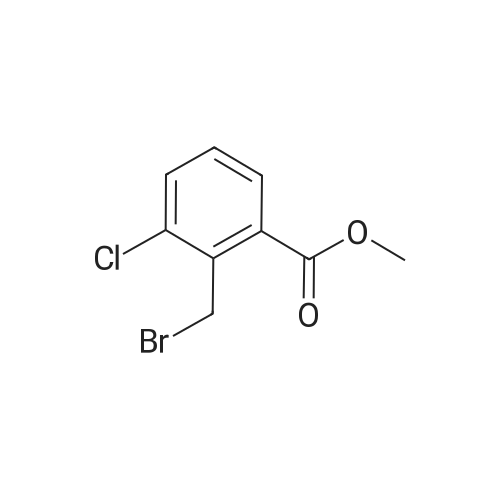
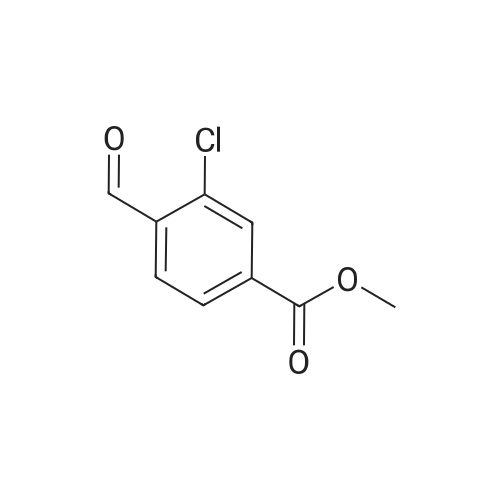
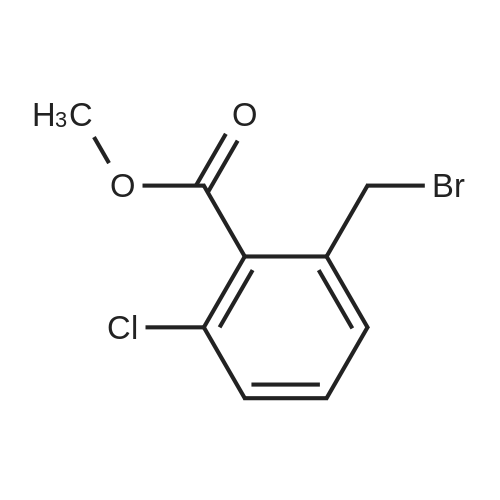
To a solution of methyl 3-chloro-4-methylbenzoate (5.0 g, 27.1 mmol) in carbon tetrachloride (50 ml) were added NBS (5.8 g, 32.0 mmol) and AIBN (0.442 g, 2.70 mmol).. The mixture was stirred at reflux for 18 h.. The mixture was allowed to cool to room temperature and then concentrated in vacuo.. The residue was purified by flash chromatography on silica (eluant EtOAc:pet. ether 0:100 to 5:95); yield 5.96 g (84%). |
| 75% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; for 4h;Heating / reflux; |
step 1-A mixture of 184a (2 mmol), NBS (2.2 mmol) and AIBN (100 mg) in CCl4 (20 mL) was heated at reflux for 4 h. The mixture was cooled, filtered and the filtrate was concentrated in vacuo. The residue was purified by SiO2 chromatography to afford 0.395 g (75%) of 184b. |
| 5.96 g (84%) |
With N-Bromosuccinimide; azobisisobutyronitrile; In tetrachloromethane; |
A1. Methyl 4bromomethyl-3-chlorobenzoate To a solution of methyl 3-chloro-4-methylbenzoate (5.0 g, 27.1 mmol) in carbon tetrachloride (50 ml) were added NBS (5.8 g, 32.0 mmol) and AIBN (0.442 g, 2.70 mmol). The mixture was stirred at reflux for 18 h. The mixture was allowed to cool to room temperature and then concentrated in vacuo. The residue was purified by flash chromatography on silica (eluant EtOAc:pet. ether 0:100 to 5:95); yield 5.96 g (84%). |
|
With N-Bromosuccinimide;benzoic peroxyanhydride; In tetrachloromethane; |
(2) Preparation of methyl 4-bromomethyl-3-chlorobenzoate Methyl 4-methyl-3-chlorobenzoate (4.5 g, 0.024 mol) was dissolved in carbon tetrachloride (100 ml), and thereto were added N-bromosuccinimide (4.8 g, 0.026 mol) and a catalytic amount of perbenzoic anhydride. The mixture was heated and refluxed under nitrogen atmosphere for 2 hours, allowed to cool, and the precipitate was separated by filtration. The filtrate was concentrated, and separated and purified by using a silica-gel column chromatography (ethyl acetate:n-hexane=1:20) to give the title compound (4.7 g, 0.018 mol) (yield 75%). NMR (CDCl3) delta: 8.04 (s, 1H), 7.91 (bd, J=8.13Hz, 1H), 7.50 (d, J=7.92Hz, 1H), 4.58 (s, 2H), 3.92 (s, 3H) |
| 5.96 g (84%) |
With N-Bromosuccinimide; azobisisobutyronitrile; In tetrachloromethane; |
A1. Methyl 4-bromomethyl-3-chlorobenzoate To a solution of methyl 3-chloro-4-methylbenzoate (5.0 g, 27.1 mmol) in carbon tetrachloride (50 ml) were added NBS (5.8 g, 32.0 mmol) and AIBN (0.442 g, 2.70 mmol). The mixture was stirred at reflux for 18 h. The mixture was allowed to cool to room temperature and then concentrated in vacuo. The residue was purified by flash chromatography on silica (eluant EtOAc:pet. ether 0:100 to 5:95); yield 5.96 g (84%). |
|
With N-Bromosuccinimide;dibenzoyl peroxide; In tetrachloromethane; for 6h;Reflux; |
(2-Chloro-4-(methoxycarbonyl)benzyl)triphenylphosphonium bromideMethyl 3-chloro-4-methylbenzoate (2.20 g, 11.96 mmol) was dissolved in carbon tetrachloride (30 mL) and N-bromosuccinimide (2.10 g, 11.80 mmol) was added followed by a catalytic amount of benzoyl peroxide (25 mg). The reaction mixture was refluxed for 6h. (ca. 90% conversion). After cooling to room temperature, a precipitate was filtered. The filtrate was concentrated to give crude brominated intermediate (3.20 g), which was used for the next step without further purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 6h;Reflux; |
Methyl 3-chloro-4-methylbenzoate (2.20 g, 11.96 mmol) was dissolved in carbon tetrachloride (30 mL) and N-bromosuccinimide (2.10 g, 11.80 mmol) was added followed by a catalytic amount of benzoyl peroxide (25 mg). The reaction mixture was refluxed for 6 h. (ca. 90% conversion). After cooling to room temperature, a precipitate was filtered. The filtrate was concentrated to give crude brominated intermediate (3.20 g), which was used for the next step without further purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; at 80℃; |
Methyl 3-chloro-4-methylbenzoate (0.30 mL, 2.0 mmol) was dissolved in carbon tetrachloride (5 mL), N-bromosuccinimide (0.39 g, 2.2 mmol) and benzoyl peroxide (48 mg, 0.20 mmol) were added, and the mixture was stirred at 80 C. overnight. The reaction mixture was filtered and concentrated under reduced pressure, 8 mol/L ammonia-methanol solution was added to the obtained residue, and the mixture was stirred at room temperature for 90 min. The reaction mixture was concentrated under reduced pressure, and the obtained residue was dissolved in dichloromethane (5 mL). A-1 (0.36 g, 1.2 mmol), WSC hydrochloride (0.29 g, 1.5 mmol) and HOAt (0.16 g, 1.2 mmol) were added, and the mixture was stirred at room temperature overnight. The reaction mixture was concentrated under reduced pressure, and the obtained residue was purified by reversed-phase high performance liquid chromatography (water-acetonitrile, each containing 0.1% trifluoroacetic acid) to give the title compound (0.40 g, 0.84 mmol, 42%). |
|
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; at 100℃; for 15h; |
To a solution of methyl 3-chloro-4-methyl-benzoate (22.5 g, 121 mmol) and 2,2- azobisisobutyronitrile (2.00 g, 12.2 mmol) in carbon tetrachloride (300 mL) was added 1- bromopyrrolidine-2,5-dione (23.8 g, 134 mmol) portion wise. The mixture was heated to 100 C and stirred at 100 C for 15 hours. On completion, the mixture was concentrated in vacuo to give a solid. The solid was washed with water (200 mL) and extracted with DCM (2 X 150 mL). The combined organic layer was dried over anhydrous sodium sulfate, filtered and concentrated in vacuum to give a residue. The residue was purified with silica gel chromatograph (petroleum ether: ethyl acetate = 100: 1) to give the title compound. NMR (400MHz, CDCh) delta = 7.99 (d, J = 1.5 Hz, 1H), 7.84 (dd, 7= 1.6, 8.0 Hz, 1H), 7.45 (d, J= 8.0 Hz, 1H), 4.53 (s, 2H), 3.86 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +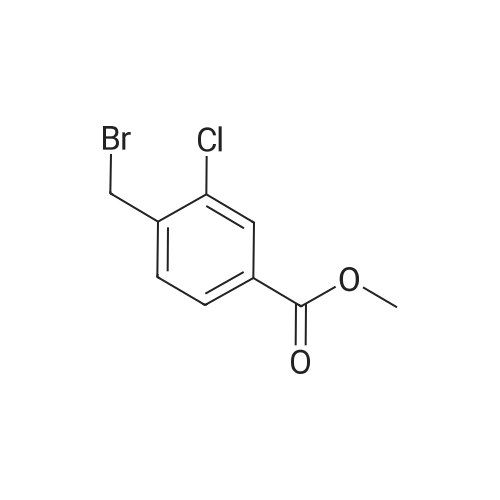

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping