PH domain-mediated autoinhibition and oncogenic activation of Akt
Bae, Hwan
;
Viennet, Thibault
;
Park, Eunyoung
, et al.
eLife,2022,11,e80148.
DOI:
10.7554/elife.80148
PubMed ID:
35968932
More
Abstract: Akt is a Ser/Thr protein kinase that plays a central role in metabolism and cancer. Regulation of Akt’s activity involves an autoinhibitory intramolecular interaction between its pleckstrin homology (PH) domain and its kinase domain that can be relieved by C-tail phosphorylation. PH domain mutant E17K Akt is a well-established oncogene. Previously, we reported that the conformation of autoinhibited Akt may be shifted by small molecule allosteric inhibitors limiting the mechanistic insights from existing X-ray structures that have relied on such compounds (Chu et al., 2020). Here, we discover unexpectedly that a single mutation R86A Akt exhibits intensified autoinhibitory features with enhanced PH domain-kinase domain affinity. Structural and biochemical analysis uncovers the importance of a key interaction network involving Arg86, Glu17, and Tyr18 that controls Akt conformation and activity. Our studies also shed light on the molecular basis for E17K Akt activation as an oncogenic driver.
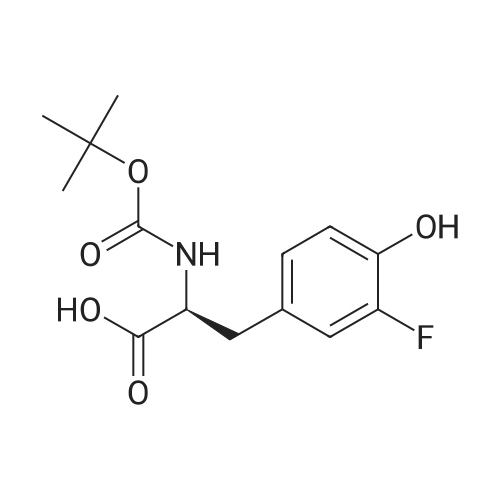
Purchased from AmBeed:
7423-96-3

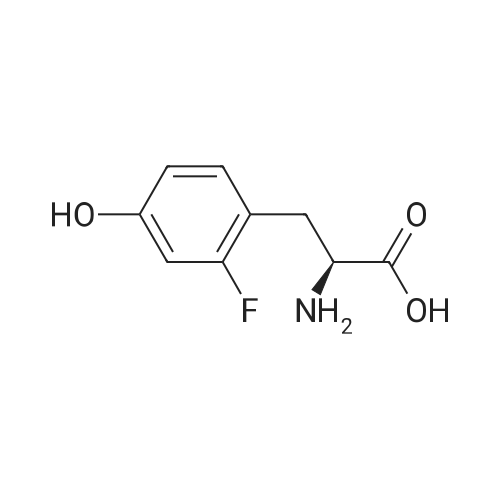
Development of a single culture E. coli expression system for the enzymatic synthesis of fluorinated tyrosine and its incorporation into proteins
Noelle M. Olson
;
Jorden A. Johnson
;
Kerstin E. Peterson
, et al.
J. Fluorine Chem.,2022,261,110014.
DOI:
10.1016/j.jfluchem.2022.110014
PubMed ID:
37197608
More
Abstract: Current experiments that rely on biosynthetic metabolic protein labeling with 19F often require fluorinated amino acids, which in the case of 2- and 3-fluorotyrosine can be expensive. However, using these amino acids has provided valuable insight into protein dynamics, structure, and function. Here, we develop a new in-cell method for fluorinated tyrosine generation from readily available substituted phenols and subsequent metabolic labeling of proteins in a single bacterial expression culture. This approach uses a dual-gene plasmid encoding for a model protein BRD4(D1) and a tyrosine phenol lyase from Citrobacter freundii, which catalyzes the formation of tyrosine from phenol, pyruvate, and ammonium. Our system demonstrated both enzymatic fluorotyrosine production and expression of 19F-labeled proteins as analyzed by 19F NMR and LC-MS methods. Further optimization of our system should provide a cost-effective alternative to a variety of traditional protein-labeling strategies.
Keywords:
Fluorine NMR ;
Fluorotyrosine ;
Metabolic labeling ;
Bromodomain ;
Tyrosine phenol lyase
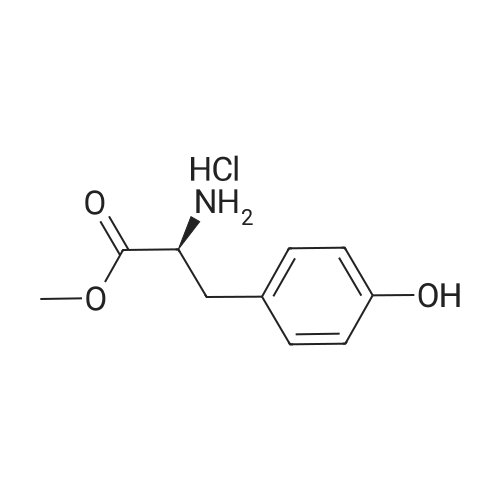
Purchased from AmBeed:
7423-96-3


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping