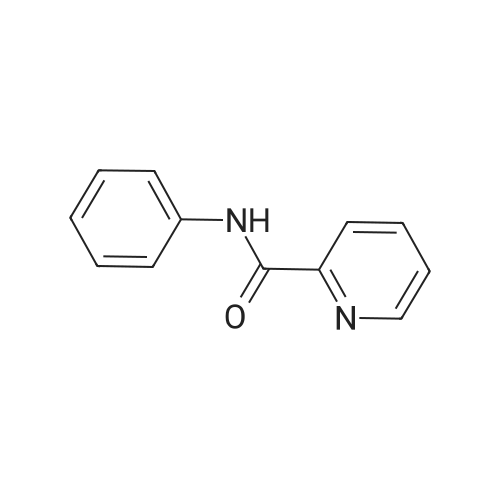
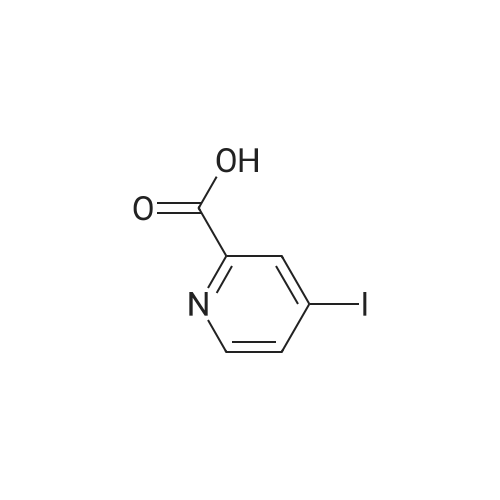
| 66% |
|
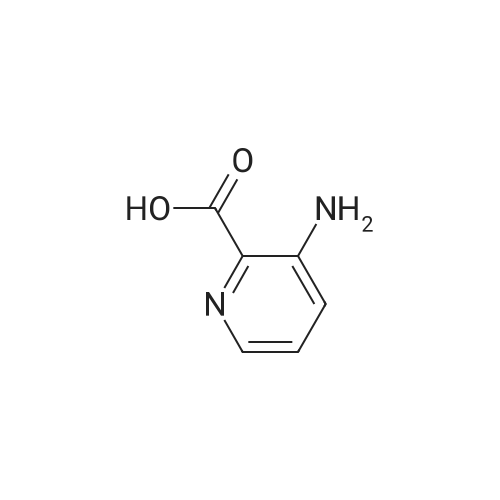
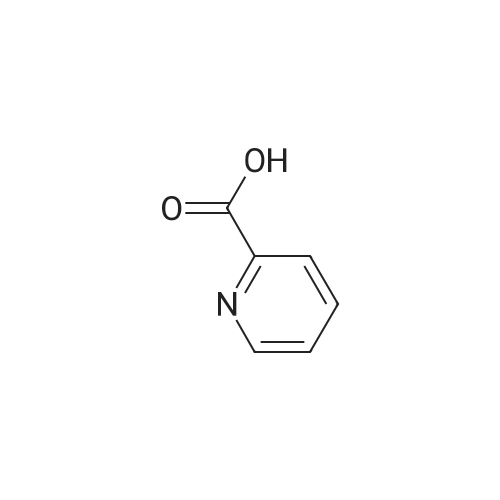
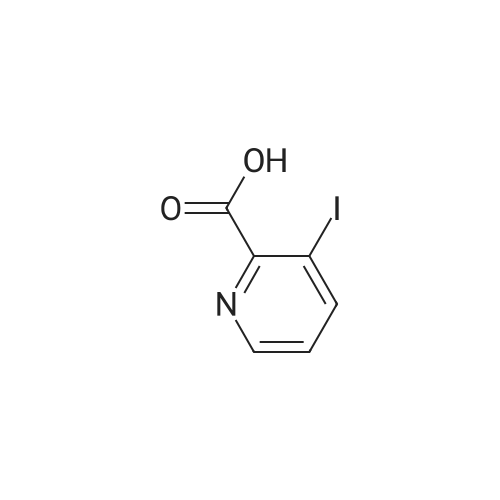
A solution of 2,2,6,6-tetramethylpiperidine (8.48 g, 60 mmol) in tetrahydrofuran (100 mL)Cooling to -78 deg C, N-butyllithium (2.5 mol / L, 16 mL, 40 mmol) was added dropwise, Slowly warmed to room temperature for 30 minutes. Cooling to -78 deg C, This was added dropwise to a suspension of pyridine-2-carboxylic acid (2.46 g, 20 mmol) in tetrahydrofuran, After completion of the dropwise addition, the reaction was allowed to proceed at room temperature for 30 minutes, Cooling to -30 deg C, A solution of iodine (15.23 g, 60 mmol) in tetrahydrofuran was added dropwise to the reaction flask, The mixture was stirred at room temperature for 1 hour, add water, Standing overnight Precipitation of solids, The target compound was filtered (3.3 g, yield 66percent). |
| 41% |
|
Description 1; 3-Iodo-2-pyridinecarboxylic acid (D1); To a stirred solution of 2,2,6,6-tetramethylpiperidine (20 ml, 0.122 mol) in dry THF (100 ml) at -78° C., under argon was added n-butyllithium (52 ml, 0.163 mol, 2.5M solution in hexanes) dropwise, followed 15 min later by a solution of 2-pyridinecarboxylic acid (5.0 g, 0.0407 mol) in dry THF (30 ml). After 10 min at -78° C., the reaction mixture was warmed to 0° C. for 30 min. and then transferred to a solution of iodine (30.9 g, 0.243 mol) in dry THF (70 ml) at 0° C., under argon. After 15 min at 0° C. the reaction mixture was warmed to 25° C. and stirred for 1 h. After this period water (80 ml) was added and the reaction mixture concentrated in vacuo. The residue was re-dissolved in water (100 ml) and washed with EtOAc (100 ml). The aqueous layer was separated, concentrated in vacuo and the resulting residue triturated with diethyl ether. The solid material was filtered and dried in vacuo before being re-dissolved in MeOH (200 ml). To this solution was added Amberlyte IR-120 ion-exchange resin (100 g) and the reaction mixture stirred at 25° C. for 2 h. After this period the resin was filtered off and the solvents concentrated in vacuo to afford the title compound (4.15 g, 41percent). deltaH (DMSO-d6, 250 MHz) 6.79 (1H, bs) 7.28 (1H, dd), 8.37 (1H, dd), 8.58 (1H, dd). MS (ES): C6H41NO2 requires 249. found (M-H+) 248. |
| 41% |
|
Description 1; 3-lodo-2-pyridinecarboxylic acid (D1);To a stirred solution of 2,2,6,6-tetramethylpiperidine (20ml, 0.122mol) in dry THF (100ml) at -78 °C, under argon was added /7-butyllithium (52ml, 0.163mol, 2.5M EPO <DP n="40"/>solution in hexanes) dropwise, followed 15min later by a solution of 2- pyridinecarboxylic acid (5.Og, 0.0407mol) in dry THF (30ml). After 10min at -78 0C, the reaction mixture was warmed to 00C for 30 min. and then transferred to a solution of iodine (30.9g, 0.243mol) in dry THF (70ml) at 00C, under argon. After 15min at O0C the reaction mixture was warmed to 25°C and stirred for 1h. After this period water (80ml) was added and the reaction mixture concentrated in vacuo. The residue was re-dissolved in water (100ml) and washed with EtOAc (100ml). The aqueous layer was separated, concentrated in vacuo and the resulting residue triturated with diethyl ether. The solid material was filtered and dried in vacuo before being re- dissolved in MeOH (200ml). To this solution was added Amberlyte IR-120 ion- exchange resin (100g) and the reaction mixture stirred at 25°C for 2h. After this period the resin was filtered off and the solvents concentrated in vacuo to afford the title compound (4.15g, 41percent). deltaH (DMSO-d6, 250MHz) 6.79 (1H, bs) 7.28 (1H, dd), 8.37 (1 H, dd), 8.58 (1H, dd). MS (ES): C6H4INO2 requires 249; found (M-H+) 248. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping