| 88% |
|
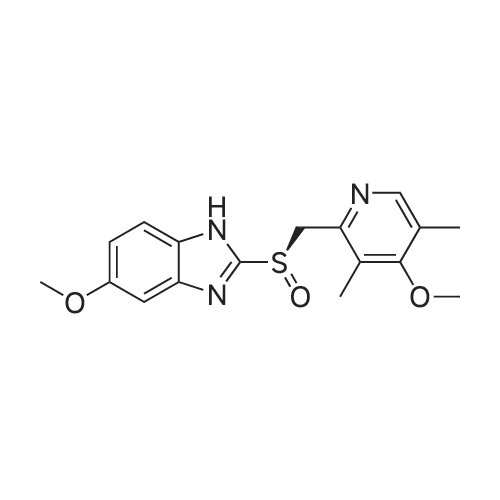
10 g of <strong>[73590-85-9]omeprazole sulfide</strong>,8.6gTetraisopropyl titanate50 ml of toluene,Stirring at 30 ° C to dissolve and clarify.Cooling to 25 ,Insulated drop of 9.2gHydrogen peroxide cumene,60 minutes drop finished,After the drop,25 holding temperature 4h.The organic solvent was distilled off under reduced pressure,The residue was washed with acetone,filter,dry,To give 9.2 g of a white solid,The molar yield was 88percentChromatography purity 99.35percentThe content of peroxide impurity in formula III is 0.52percent. |
| 88.4% |
|
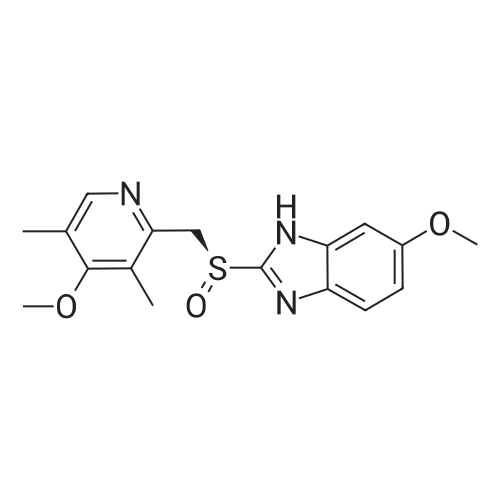
Omeprazole sulfide 6.58g (0.02mol)Soluble in 100ml of absolute ethanol,Adjust to pH=8-9 with triethylamine.240 mg of graphene oxide containing Ti4 + mass 4.0percent was added.Stir at 40 ° C for 10 min,3.4 g (0.03 mol) of 30percent hydrogen peroxide was slowly added dropwise.After the dropwise addition, the reaction was carried out for 3 h. The catalyst was filtered off.And washed three times,The collected filtrate was concentrated to give a crude omeprazole.Then beat with ethyl acetate and filter by suction.Drying to get omeprazole 6.1g,The yield was 88.4percent. |
| 79.1% |
With m-chloroperoxybenzoic acid; In ethyl acetate; |
EXAMPLE 1 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]benzimidazole (10 g; 0.0304 mole) was suspended in ethyl acetate (100 ml) and cooled below 0° C. 3-chloroperoxybenzoic acid (5.25 g; 0.0304 mole) was added in such a manner that the temperature did not exceed 5° C. After completed addition it was left to crystallize for another half an hour at a temperature below 5° C. The product formed was filtered off, washed with ethyl acetate and dried in vacuo. Crude omeprazole (8.3 g; 79.1percent) was obtained. |
| 76.2% |
With m-chloroperoxybenzoic acid; sodium carbonate; In ethyl acetate; |
EXAMPLE 2 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]benzimidazole (10 g; 0.0304 mole) was suspended in ethyl acetate (100 ml) and cooled below 0° C. 3-chloroperoxybenzoic acid (5.25 g; 0.0304 mole) was added in such a manner that the temperature did not exceed 5° C. After the completed addition it was stirred for half an hour, the cooling was removed, a 4percent sodium carbonate solution (40 ml) was added and it was stirred for another half an hour. The product was filtered off and washed with water. After drying in vacuo crude omeprazole (8.0 g; 76.2percent) was obtained and it was purified according to the process disclosed in Example 1. |
| 75% |
With titanium(IV) isopropylate; D-tartaric acid; Cumene hydroperoxide; N-ethyl-N,N-diisopropylamine; In toluene; at 20 - 65℃; for 1h; |
In a 500 mL three-necked flask,A solution of 5-methoxy-2- (4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole(14.8 g, 45 mmol) was added to 75 mL of toluene, the temperature was raised to 65 ° C,Dissolved after addingD-tartrate(5.7 g, 27.5 mmol)And tetraisopropyl titanate(3.9 g, 13.8 mmol),While adding a small amount of purified water,After stirring for 1 hour,Down to 10 ° C. N, N-diisopropylethylamine (1.6 g, 12 mmol)And toluene-diluted cumene hydroperoxide (8 g, 45 mmol) were added and the reaction was continued at 20 ° C for 1 hour. After completion of the reaction, ammonia (60 mL) and purified water were added and stirred for 20 minliquid. The aqueous layer was added to dichloromethane (150 mL) and stirred for 20 min. The dichloromethane layer was added anhydrous sodium sulfate (15 g)Dried and dried for 1 hour, then filtered, and the dichloromethane was removed by steaming under reduced pressure at 50 ° C,The oil is an esomeprazole, Product yield 75percent. |
| ~ 69% |
With dihydrogen peroxide;sodium tungstate; In ethanol; water; at 20℃; for 6.5h;Product distribution / selectivity; |
CONTROL EXAMPLE 2; 0.35 g of 2-[[(3,5-dimethyl-4-methoxy-2-pyridinyl)methyl]thio]-5-methoxy-1H-benzimidazole was suspended in 5 ml of ethanol at room temperature. 0.035 g of Na2WO4*2H2O oxidation catalyst was dissolved in 0.099 g H2O2 (35percent aqueous solution), and further diluted with 2 ml of water. The oxidant/catalyst solution was added to the reactant suspension dropwise so that the addition was completed in about 30 minutes while stirring at room temperature. The reaction was continued for additional 6 hours while stirring. 2 ml of 10percent Na2S2O3 aqueous solution was then, added, and the resulting mixture was subjected to a reduced pressure to remove ethanol therefrom. Finally, a precipitate was formed after adding a diluted acetic acid aqueous solution to a pH value of about 8, which was filtered, water washed, and dried in vacuo to obtain Omeprazole with a yield of about 69percent (LC purity>80percent). |
| 63 - 79% |
With tert.-butylhydroperoxide;bis(acetylacetonate)oxovanadium; In ethanol; water; at 16 - 17℃; for 3.08333h; |
1.5 mg (0.6percent molar) VO(acac)2) was dissolved in 12 ml ethanol at room temperature. The solution was stirred and 3 grams of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]benzimidazole (MPB) were added. 1.5 ml aqueous tert-butyl hydroperoxide (TBHP) (70percent) was added over a 5-minute period at 16-17° C. and the solution was then stirred for 3 hours. After completion of the reaction, the product mixture was cooled to about 15° C. and treated with aqueous sodium metabisulphate. The resultant solid was filtered off, washed with cooled ethyl acetate to afford the end product as an almost white solid (2.5 grams, yield 79percent). |
| 32% |
With dihydrogen peroxide;bis(acetylacetonate)oxovanadium; In ethanol; water; at 20℃; for 12h; |
4 mg (0.06percent molar) VO (acac)2 were added to suspension of 9 grams of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]-1H-benzimidazole (MPB) in 66 ml ethanol at room temperature. 35 ml of 35percent aqueous hydrogen peroxide (150percent mol) was added at room temperature with no visible exotherm, the mixture was then stirred. After 12 hours the reaction mixture still contained 65percent of untreated MPB and only 32percent omeprazole. Prolongation of the reaction time did not lead to further production of omeprazole. |
|
With sodium hydroxide; sodium hypochlorite; In dichloromethane; water; at -5 - 0℃; for 3h; |
((5-METHOXY-2-[[(4-METHOXY-3, 5-DIMETHYL-2-PYIRDYL) METHYL]-THIO]-LH-BENZIMIDAZOLE, (20g) was suspended in 200ML of dichloromethane. 140g of sodium hypochlorite solution (chlorine content: 3.6-4. 2percent ; sodium hydroxide content: 2.8-3. 0 percent) was added over a period of 3 hours maintaining a temperature OF-5°C to 0°C. The organic layer was separated and extracted with 200ml of 5percent sodium hydroxide solution. The pH of the aqueous layer was adjusted to between 8-8.5 using dilute acetic acid. The solids were filtered, washed with chilled water and dried in an oven to give 17g of the title compound. |
|
With peracetic acid; In ethyl acetate; |
Example 4 Preparation of Omeprazole To a solution of 100 g of the product from Example 3 in ethyl acetate (500 ml) was added peracetic acid (125 g) slowly at a temperature of -25°C. The contents were further stirred for 30 minutes and the pH of the mixture was adjusted to 8 with 10percent aqueous sodium hydroxide. The product thus precipitated was filtered and washed with water. The crude product was purified from methanol to afford 85g of pure omeprazole. |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 5℃; for 0.333333 - 0.416667h; |
EXAMPLE 3; Preparation of 5/6-methoxy-1benzyloxycarbonyl-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazoleTo a solution of 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole (30 g) in dichloromethane (165 mL) at 0-5° C., under an inert atmosphere, was added meta-chloroperbenzoic acid (0.95 eq) over 10 minutes. The mixture was stirred for 10-15 minutes. To the reaction was added 12percent ammonium hydroxide (180 mL). The layers were separated. The organic layer was extracted with 12percent ammonium hydroxide (2.x.180 mL). The combined aqueous layers were washed with toluene (90 mL). To the aqueous layer was added dichloromethane (120 mL) and the mixture was cooled to 0-5° C. The pH was adjusted to pH=8.5-9.5 using 50percent aqueous acetic acid. The layers were separated. The aqueous layer was extracted with dichloromethane (2.x.90 mL). The combined organic layers were washed with brine (30 mL), dried over sodium sulfate, filtered through celite and vacuum distilled to 150 mL to give a solution of 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulphinyl]1H-benzimidazole in dichloromethane. |
|
With trans-stilbene ozonide; In methanol; for 3.5h;Heating / reflux;Product distribution / selectivity; |
Trans-stilbene ozonide was prepared following the recipe of example 1, in an amount of 0.031 mol.The thus-prepared ozonide was added drop-wise over 0.5h to a boiling solution of sulfide (0.031 mol) in 100 ml of methanol. The reaction solution is boiled for 3 hours under reflux conditions. The experiment was performed for a number of sulfides, generally represented by formula III. After 3 hours, the reaction mixtures were analyzed for conversion rate. The results are shown in table 1. In all cases, sulfoxide selectivity was 100percent. |
|
With 1-hexen-ozonide; In methanol; for 3.5h;Heating / reflux;Product distribution / selectivity; |
21 g 1-Hexene (0.20 mol) in 500 ml of methanol at -15°C was converted with 1.2 molar equivalents of ozone, thus producing 1-hexen-ozonide. The ozonide was present as a clear solution in methanol.The solution was used directly for the oxidation of the sulfides represented by formula III. Thereto, the ozonide was added dropwise over 0.5h to the boiling solution of the sulfide (0.20 mol) in 200 ml methanol. The reaction solution was refluxed for 3 hours. After 3 hours, the reaction mixtures were analyzed for conversion rate. The results are shown in table 2. In all cases, sulfoxide selectivity was 100percent. |
|
With dihydrogen peroxide;sodium molybdate; In water; acetic acid; ethyl acetate; at 5 - 12℃; for 6.75h;pH 6 - 6.5; |
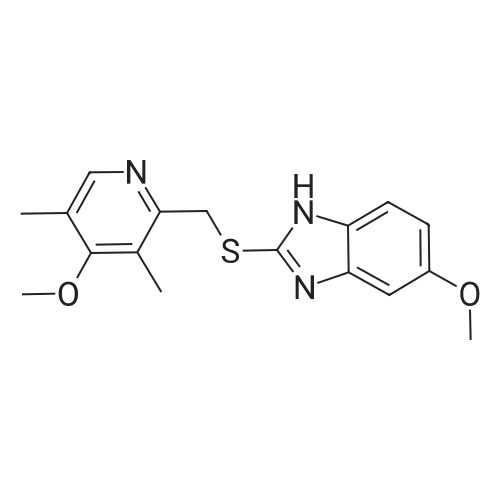
2-(chloromethyl)-3,5-dimethyl-4-methoxy pyridine hydrochloride (IV) (100.0 g) and 2-mercapto-5-methoxy benzimidazole (III) (81.0 g) were taken in RBF. Ethyl Acetate (400 mL) was added to RBF at 25°C to 350C. Sodium hydroxide (50.0 g) solution in water (200 mL) was added to the reaction mass within 30 mins. The reaction0 mass was stirred for 1 hr and heated to 500C to 55°C for 1 hr. After completion of the reaction on TLC, reaction mass was cooled to 250C to 300C. Water (200 mL) was added and stirred to separate the organic and aqueous layers. Aqueous layer was extracted with ethyl acetate (150 mL) and separated. The combined ethyl acetate layer was charcaolised (5.0 g) and stirred for 30 mins. The reaction mass was filtered through hyflow bed and washed with ethyl acetate (50 mL). The pH of the organic layer was adjusted to about 6.0 to 6.5 with acetic acid (0.5 mL) and cooled to 5°C to 100C.Sodium molybdate (1.33 g) solution in water (13.2 mL) was added to the reaction mass and stirred for 15 mins. 50percent hydrogen peroxide (37.0 g) was added into the reaction mass within 1.5 hrs at 5°C to 12°C. The reaction mass was stirred for 5 hrs.After completion of the reaction on TLC, the reaction mass is treated with sodium thiosulphate (11.0 g) solution in water (11.0 mL). Sodium hydroxide (6.0 g) solution in water (6 mL) was added into the reaction mass to adjust the pH of about 7.0 to 7.5. The reaction mass was further cooled to 00C to 5°C and stirred for 60 mins. The product was filtered and washed with mixture of methanol (50 mL) and water (50 mL) followed by washing with chilled ethyl acetate (75 mL).Crude omeprazole (120.0 g) wet-cake as obtained above and methanol (160 mL) were taken in another RBF at 25°C to 35°C. Sodium hydroxide (15.8 g) solution in water (176 mL) was added into the reaction mass. Charcaol (2.6 g) was added and stirred for 30 mins. The reaction mass was filtered on hyflow bed and washed with mixture of methanol (10 mL) and water (10 mL). The filtrate was treated with sodium hydrosulphite (2.0 g). The reaction mass was slowly treated with acetic acid (22.5 mL) to adjust the pH of about 7.5 to 7.9. The product was filtered and washed with water(244 mL) and dried at 400C to 45°C to obtain 100.0 g crystalline omeprazole Form B. Yield 68percent based on input 2-(chloromethyl)-3,5-dimethyl-4-methoxy pyridine hydrochloride (IV).HPLC purity: 99.87percentIndividual Impurities are as under:Im purity- A at RRT 0.44 : 0.01percent Impurity-B at RRT 0.46 : Not detectedImpuriry-C at RRT 0.80 : 0.02percentImpurity-D at RRT 0.90 : Not detectedImpurity-E at RRT 3.26 : Not detectedUnk Impurity: 0.03percent Total Impurities : 0.13percentImpurity-A: 5-methoxy- 1 H-benzimidazole-2-thiolImpurity-B: 2-[(R,S)]-[(3,5-dimethylpyridine-2-yl)methyl]sulphinyl]-5-methoxy-lH- benzimidazole Impurity-C: 5-methoxy-2[[4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulphanyl]- lH-benzimidazole [Omeprazole Sulfide]Impurity-D: 5-methoxy-2[[4-methoxy-3,5-dimethylpyridin-2-yl)methyl]suIphonyl]- lH-benzimidazole [Omeprazole Sulfone] Impurity-E: 4-methoxy-2-[[(R,S)-(5-methoxy- 1 H-benzimidazole-2-yl)- sulphinyl]methyl)-3,5-dimethylpyridine- 1 -oxide [Omeprazole N-Oxide] |
|
With dihydrogen peroxide;bis(acetylacetonate)oxovanadium; In water; acetone; at 10 - 15℃; for 3.5h; |
Preparation of omeprazoleVanadyl acetylacetonate (4.8 gm) was added to water (210 ml) and then cooled to 0 to 10°C. To the reaction mixture was added hydrogen peroxide (50 percent, 120 ml) at 0 to 10°C and stirred for 20 minutes. 5-Methyoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2- yl)methyl]thio]-l H-benzimidazole (480 gm) and acetone (1950 ml) were added to the reaction mixture. The reaction mixture was maintained for 3 hours 30 minutes at 10 to 15°C and then added sodium hydroxide (46 percent, 16 ml) and water (1600 ml). The reaction mass was then cooled to 0 to 5°C and pH of the reaction mass was adjusted to 7.5 to 8.5 with acetic acid (1 1 gm) at 0 to 10°C. The reaction mass was stirred for 2 hours and filtered to obtain a wet solid. To the wet solid was added water (700 ml) and then added liquor ammonia (38 gm) and methanol (700 ml) at room temperature. The reaction mass was then cooled to 0 to 10°C, stirred for 45 minutes and filtered. The solid obtained was dried to give 405 gm of omeprazole. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping