| 94.8% |
With triethylamine; palladium dichloride In 1-methyl-pyrrolidin-2-one at 20 - 75℃; for 6 h; Inert atmosphere |
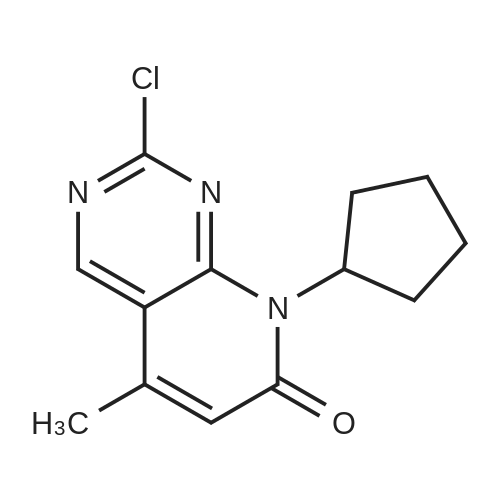
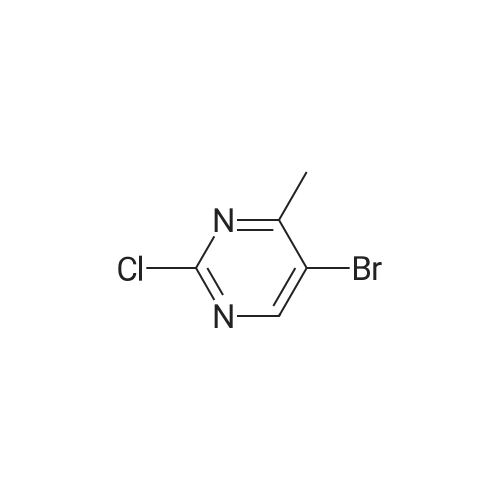
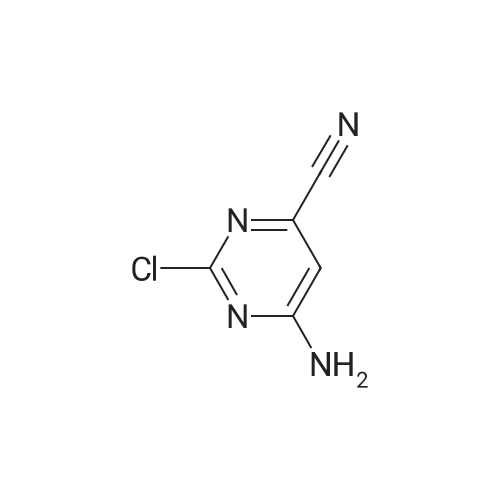
Compound 2 (10.0 g, 36.2 mmol, 1.0 eq) was added at room temperature.N-methylpyrrolidone (NMP, 50 mL),Crotonic acid (4.7 g, 54.6 mmol, 1.5 eq) and triethylamine (14.7 g, 144.9 mol, 4.0 eq).The container is passed through three times of nitrogen,PdCl 2 (0.195 g, 1.10 mmol, 0.03 eq) was added to the exhausted reaction mixture.Three more nitrogen passes.After the raw materials are stirred and dissolved at room temperature,Rapidly warm up to 75 ° C,The stirring reaction was closed for 6 h,Sampling (diluted with ethyl acetate),TLC detection (DCM: MeOH = 12:1),The starting compound 1 is completely reacted,Add acetic anhydride to react 0.5h sampling point plate,The intermediate reaction is complete,The reaction mixture will be brought to 20 ° C,Add triethylamine hydrobromide solution (100 mL),Stir for 1h,Filtering,Water washing,Get a gray solid. Add ethyl acetate to dissolve,Add anhydrous magnesium sulfate to dry the mixture,Insoluble matter (salt and insoluble catalyst) is filtered out,Wash with ethyl acetate.Concentrate the filtrate to a low volume,Add n-hexane,Beating at 65 ° C for 30 min,Cool to 20 ° C and stir for 1 h.Filtering the solid,Vacuum drying,Obtained a brownish yellow solid,That is, Compound 1 (9.04 g, yield 94.8percent, purity 99.1percent). |
| 80% |
Stage #1: With bis(benzonitrile)palladium(II) dichloride; N-ethyl-N,N-diisopropylamine; tris-(o-tolyl)phosphine In tetrahydrofuran at 75℃; for 20 h; Inert atmosphere
Stage #2: at 75℃; for 2 h; |
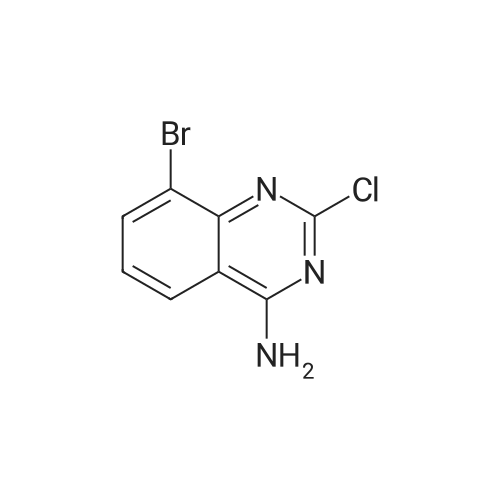
Step ii): synthesis of 2-Chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin- 7-one formula 15)Step ii): A 1 L 3-necked RBF was charged with 50.0 g of (5-Bromo-2-chloro-pyrimidin- 4-yl)-cyclopentyl-amine (0.181 mol, 1 .00 eq.), 39.0 g crotonic acid (0.452 mol, 2.5 eq.), 125 ml diisopropylethylamine (0.741 mol, 4.1 eq.) and 125 ml THF. The mixture was degassed by applying 3 vacuum/argon cycles then 1 .10 g of tri-o-tolyl-phosphine (3.62 mmol, 0.02 eq.) and 1 .39 g dichlorobis(benzonitrile)palladium(ll) (3.62 mmol, 0.02 eq.) were added. The mixture was degassed again by applying 3 vacuum/argon cycles then heated to 75°C and stirred under argon for 20 h. Then, 43.0 ml of acetic anhydride (0.452 mol, 2.5 eq.) were added and the mixture further stirred at 75^ for 2 h. The reaction was quenched with 250 ml water and the mixture allowed to cool down to RT. After 1 h stirring, 125 ml water was added while cooling to Ι δ'. The precipitated solid was collected by filtration, washed with water (125 ml), cold isopropanol (3 x 125 ml), dried in air for 10 min then at 50<€/25 mbar for 20 h to give 38.13 g of 2-Chloro-8- cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (formula 15) (80.0percent yield) as yellowish solid. HPLC (Method 1 ): 8.67 min (99.6percent) (254 nm).1H NMR (400 MHz, CDCI3, δ ppm): 1 .68 (br. s, 2 H), 1 .84 - 1 .98 (m, 2 H), 2.12 (br. s, 2 H), 2.22 (br. s, 2 H), 2.44 (s, 3 H), 5.84 (m, 1 H), 6.53 (br. s, 1 H), 8.74 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping