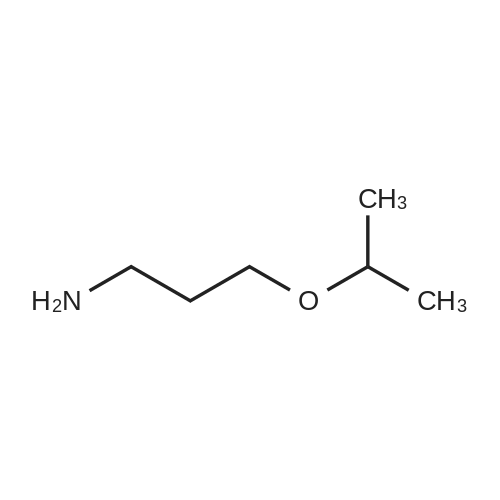
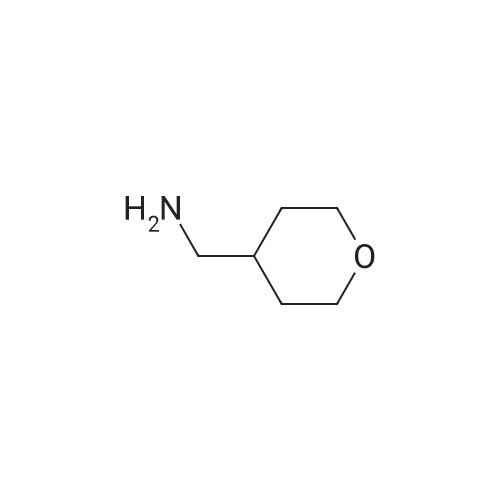
|
With trichlorophosphate; at 102℃; for 0.75h; |
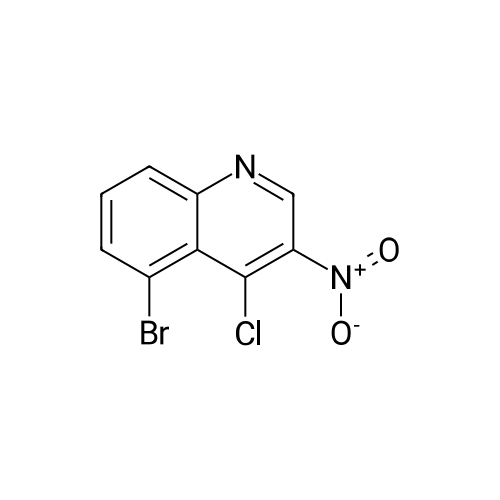
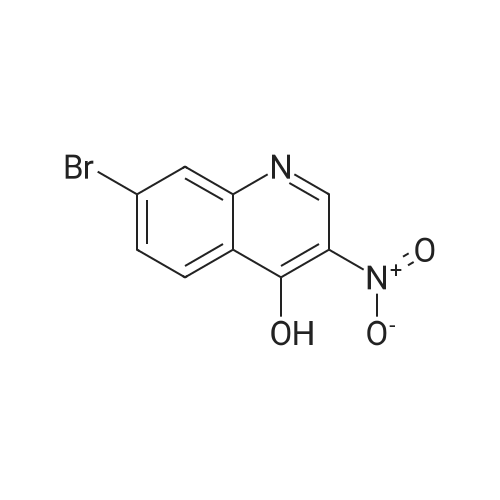
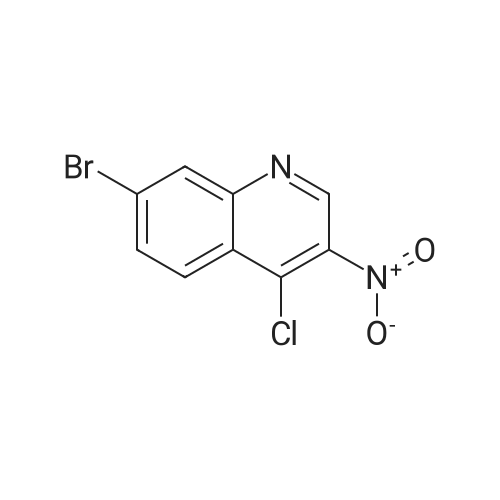
Part D 7-Bromo-3-nitroquinolin-4-ol (42 g, 156 millimoles (mmol) ) was suspended in POC13 (130 mL) and brought to 102C under an atmosphere of N2. After 45 min, all of the solids had dissolved, so the reaction was cooled to room temperature (RT). The resulting solids were collected by filtration, washed with H20, and then partitioned with CH2C12 (3 L) and 2M Na2C03 (500 mL). The organic layer was separated, washed with H20 (1x), dried over Na2S04, filtered, and concentrated to afford 33.7 g of 7-bromo-4-chloro-3- nitroquinoline as a beige solid. |
|
|
D7-Bromo-3-nitroquinolin-4-ol (42 g, 156 mmol) was suspended in POC13 (130 mL) and brought to 102 C under an atmosphere of N2. After 45 min, all of the solids had dissolved, so the reaction was cooled to room temperature. The resulting solids were collected by filtration, washed withH20, and then partitioned withCHzClz (3 L) and 2MNa2CO3 (500 mL). The organic layer was separated, washed withH20(lx), dried overNa2S04, filtered, and concentrated under reduced pressure to afford 33.7 g of 7-bromo-4-chloro-3-nitroquinoline as a beige solid. 'H NMR (300 MHz, CDC13) 8 9.26 (s, 1H), 8.41 (d, J= 1. 8 Hz, 1H), 8. 30 (d, J= 9.0 Hz, 1H), 7.90 (dd, J= 8.9, 2.1 Hz, 1H). |
|
With trichlorophosphate; at 102℃; for 0.75h; |
Part D 7-Bromo-3-nitroquinolin-4-ol (42 g, 156 mmol) was suspended in POC13 (130 mL) and brought to 102 C under an atmosphere of N2. After 45 min, all of the solids had dissolved, so the reaction was cooled to room temperature. The resulting solids were collected by filtration, washed with H20, and then partitioned with CH2C12 (3 L) and 2M Na2C03 (500 mL). The organic layer was separated, washed with H2O (lx), dried over Na2S04, filtered, and concentrated under reduced pressure to afford 33.7 g of 7-bromo-4- chloro-3-nitroquinoline as a beige solid. 'H NMR (300 MHz, CDC13) 8 9.26 (s, 1H), 8. 41 (d, J= 1. 8 Hz, 1H), 8. 30 (d, J= 9.0 Hz, 1H), 7.90 (dd, J= 8.9, 2.1 Hz, 1H). |
|
With trichlorophosphate; at 102℃; for 0.75h; |
Part D 7-Bromo-3-nitroquinolin-4-ol (42 g, 156 mmol) was suspended in POC13 (130 mL) and brought to 102 C under an atmosphere of N2. After 45 min, all of the solids had dissolved, so the reaction was cooled to room temperature. The resulting solids were collected by filtration, washed with H2O, and then partitioned with CHZCLZ (3 L) and 2M Na2C03 (500 mL). The organic layer was separated, washed with H20 (LX), dried over NA2S04, filtered, and concentrated to afford 33.7 g of 7- BROMO-4-CHLORO-3-NITROQUINOLINE as a beige solid. |
|
With N-ethyl-N,N-diisopropylamine; trichlorophosphate; at 15 - 100℃; for 16h; |
General procedure: To a mixture of 6-bromo-3-nitro-quinoline-2,4-diol (30 g, 105.24 mmol, 1 eq) in POCh (484.12 g, 3.16 mol, 293.41 mL, 30 eq) was added N,N-diisopropylethylamine (40.81 g, 315.73 mmol, 55.00 mL, 3 eq) slowly at l 5C. The mixture was stirred at 100 C for 16 hrs. The mixture was concentrated in vacuum. The residue was poured into ice water (2000 mL), filtered and washed with H2O (500 mL x 3), and dried to provide 6-bromo-2,4-dichloro-3- nitro-quinoline (30 g, 93.18 mmol, 88.54% yield) as a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping