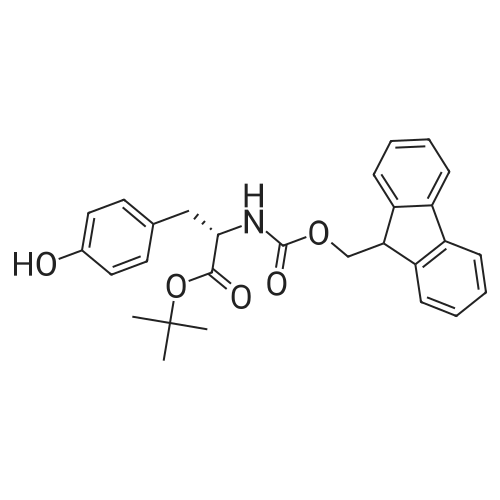
Alternatived Products of [ 71989-38-3 ]
Product Details of [ 71989-38-3 ]
| CAS No. : | 71989-38-3 |
MDL No. : | MFCD00037129 |
| Formula : |
C28H29NO5
|
Boiling Point : |
- |
| Linear Structure Formula : | C9H11NO3C4H8C15H10O2 |
InChI Key : | JAUKCFULLJFBFN-VWLOTQADSA-N |
| M.W : |
459.53
|
Pubchem ID : | 10895791 |
| Synonyms : |
|
Application In Synthesis of [ 71989-38-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 71989-38-3 ]
- 1
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
Ac-Tyr-Asp-Phe-Gly-OH
[ No CAS ]
- 2
-
Fmoc-Leu-Wang resin
[ No CAS ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
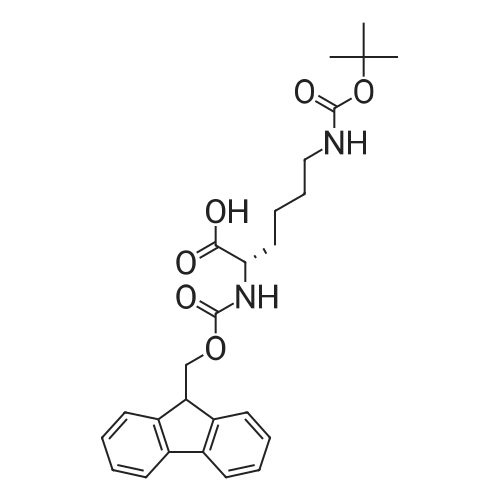 [ 71989-26-9 ]
[ 71989-26-9 ]

-
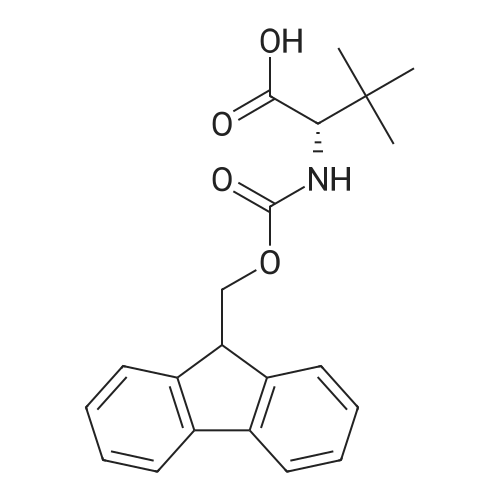 [ 132684-60-7 ]
[ 132684-60-7 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
C43H69N9O9
[ No CAS ]
- 3
-
Fmoc-Leu-Wang resin
[ No CAS ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
 [ 1198076-80-0 ]
[ 1198076-80-0 ]
- 4
-
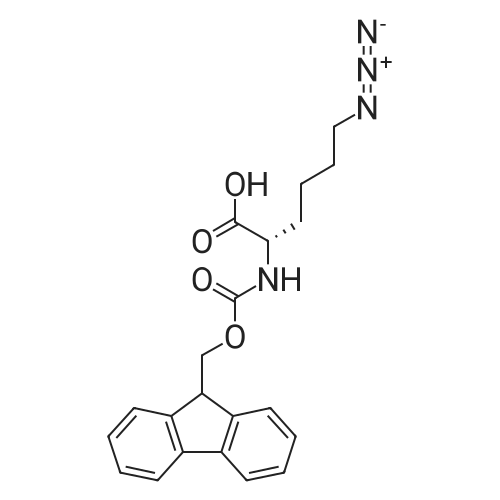 [ 159610-89-6 ]
[ 159610-89-6 ]

-
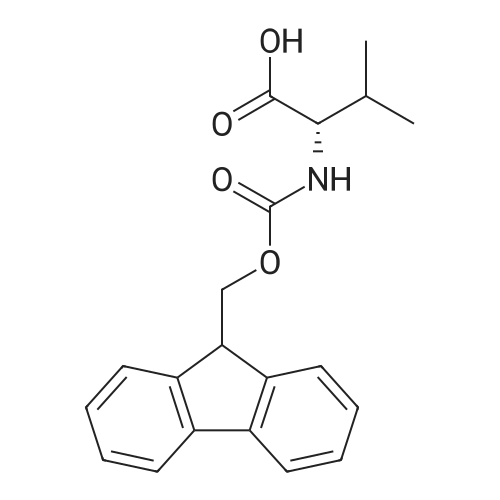 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
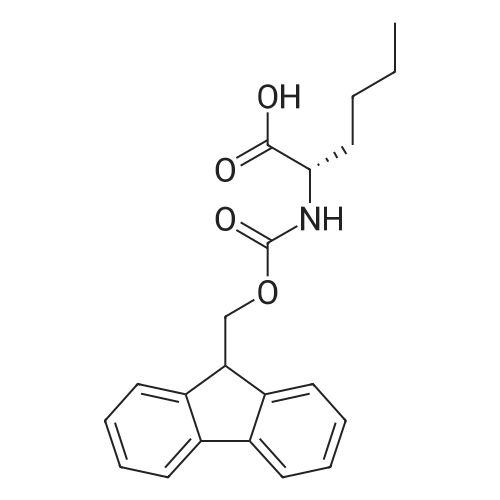 [ 77284-32-3 ]
[ 77284-32-3 ]

-
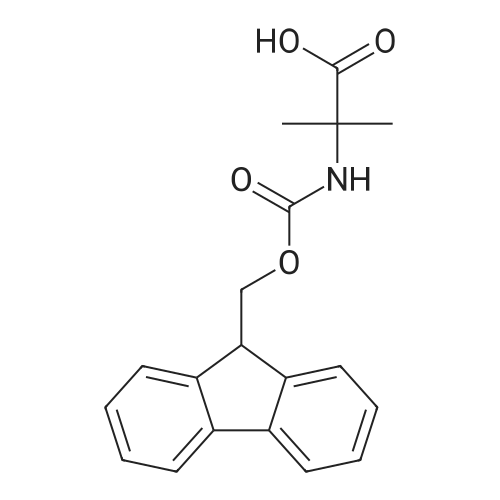 [ 94744-50-0 ]
[ 94744-50-0 ]

-
N,N'-bis(tert-butyloxycarbonyl)-L-lysine dicyclohexylamine salt
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
 [ 1443329-49-4 ]
[ 1443329-49-4 ]
- 5
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
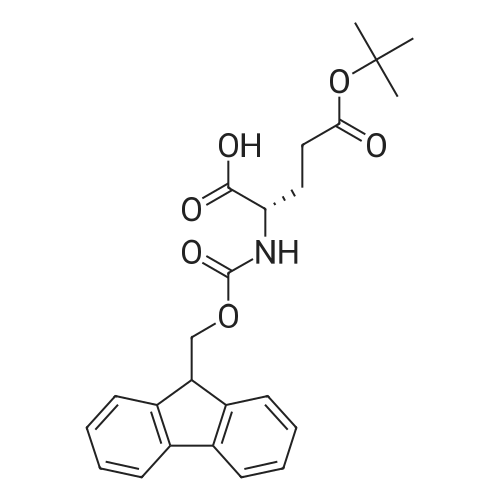 [ 71989-18-9 ]
[ 71989-18-9 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
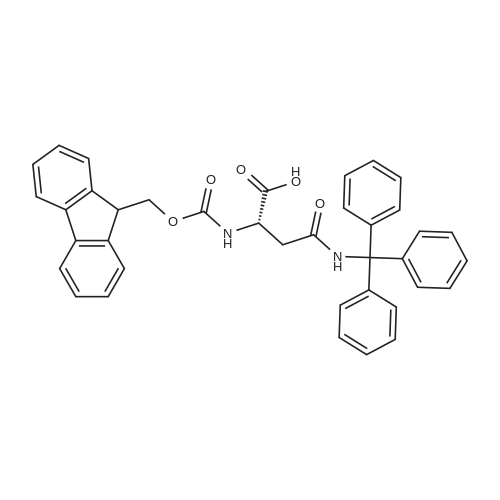 [ 132388-59-1 ]
[ 132388-59-1 ]

-
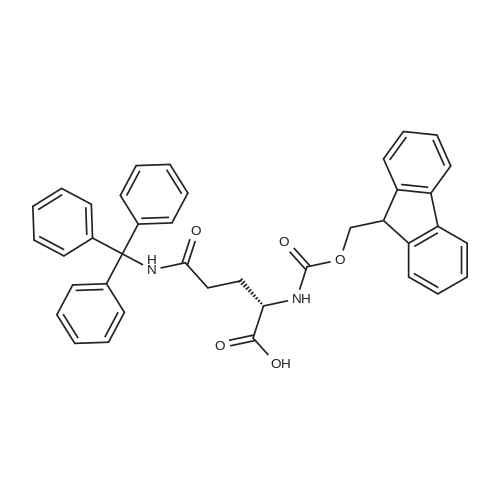 [ 132327-80-1 ]
[ 132327-80-1 ]

-
(S)-6-[(Diphenyl-p-tolyl-methyl)-amino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoic acid
[ No CAS ]

-
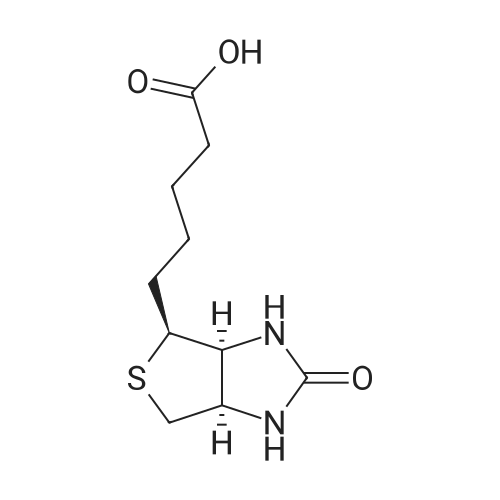 [ 58-85-5 ]
[ 58-85-5 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
C66H94N20O21S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
General procedure: 4.1.1. Peptide synthesis; 4.1.2; Solid-phase peptide synthesis (SPPS) was performed with standardFmoc chemistry on rink amide resin using an automated peptidesynthesizer (Syro I, Multisyntech). The resin was loaded into a5 mL reactor with a frit at the bottom. Swelling was performed bydispensing 1 mL DMF and incubating for 15 min (2) with 10 sshaking every minute. Fmoc deprotection was achieved by treatmentwith 40percent piperidine DMF for 3 min and 20percent piperidine inDMF for 12 min (10 s/min shaking). Peptide couplings were carriedout by double couplings with Fmoc-protected amino acids(5 equiv), HBTU (5 equiv), HOBt (5 equiv) and DIPEA (10 equiv) inDMF for 40 min (10 s/min shaking). At the respective position,Fmoc-F2Pmp-OH (3 equiv) was coupled in DMF (1 mL) by manualaddition using TBTU (3 equiv), HOBt (3 equiv) and DIPEA (6 equiv)for 3 h, after 3 min preactivation. In case of the sequences for which side-chain labeling with biotinor carboxyfluorescein was planned, an additional 4-methyltrityl-(Mtt-) protected lysine was coupled to the N-terminus. Toselectively remove the Mtt group the resin was washed for 1 minwith DCM (3), deprotection was then achieved by treatment with1.8percent TFA in DCM for 3 min (10). During the deprotection the DCMsolution turned yellow.For fluorescein-labeling of the amine side-chain 5(6)-carboxyfluorescein(3 equiv), HATU (3 equiv), HOAt (3 equiv) andDIPEA (6 equiv) were dissolved in DMF and pre-activated for3 min. The solution was aspirated and coupling was allowed toproceed for 1 h. This step was repeated 4 times.For biotin-labeling of the amine side-chain the resin waswashed for 1 min in NMP (3). D-(+)-Biotin (3 equiv), HATU(3 equiv), HOAt (3 equiv) and DIPEA (6 equiv) were dissolved inNMP and pre-activated for 3 min. The solution was aspirated andcoupling was allowed to proceed for 2 h. This step was repeated2 times. N-terminal acetylation (where applicable) was achieved by dispensing800 lL of a mixture of acetic anhydride/pyridine (1:9) andreaction twice for 5 min (10 s/min shaking). After each deprotection,coupling or acetylation step, 5 washings (1 min each) withDMF were performed (10 s/min shaking).After synthesis the resin was transferred in a 5 mL syringeequipped with a frit, washed with DCM for 1 min (3) and driedin high vacuum for at least 30 min. For cleavage 1 mL of a mixtureof TFA and TIS (20:1) was added. The syringe with the mixture waskept on a shaker for 3 h. Then the liquid phase was filtered into20 mL of ice-cold Et2O. Formed precipitate was centrifuged,washed with ice-cold Et2O (2 20 mL) and purified by HPLC. 4.1.2. Azide functionalization of the N-terminus; To the peptides with the longer carbon linker, 6-azidohexanoicacid was coupled (with standard coupling conditions) to the Nterminalamine.The N-terminal amine of the peptides with the shorter linkerwas converted to an azide functionality directly on solid support.Using the compound imidazole-1-sulfonyl-azide*HCl (synthesissee beneath) and modified conditions, which were reported forsolution phase chemistry from Goddard?Borger and Stick:8 Theresin was washed for 1 min each with DCM (2), DCM/MeOH(2) and MeOH (3). Then (for 40 mg resin, loading= 0.62 mmole/g) 1.4 equiv of imidazole-1-sulfonyl-azide*HClin 1 mL MeOH and 100 ll of a saturated and centrifuged solutionof CuSO4*5H2O was added. After 1 min, DIPEA (1.8 equiv) wasadded and the coupling was allowed to proceed for 1 h andrepeated once more with an intermediate washing with MeOH(3 1 min). |
- 6
-
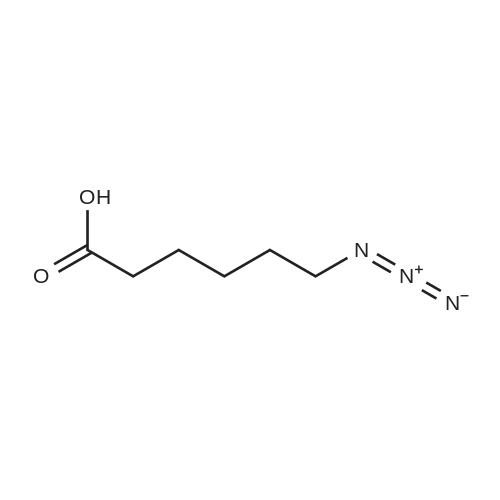 [ 79598-53-1 ]
[ 79598-53-1 ]

-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-18-9 ]
[ 71989-18-9 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
(S)-6-[(Diphenyl-p-tolyl-methyl)-amino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoic acid
[ No CAS ]

-
 [ 58-85-5 ]
[ 58-85-5 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
C72H105N21O22S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
General procedure: 4.1.1. Peptide synthesis; 4.1.2; Solid-phase peptide synthesis (SPPS) was performed with standardFmoc chemistry on rink amide resin using an automated peptidesynthesizer (Syro I, Multisyntech). The resin was loaded into a5 mL reactor with a frit at the bottom. Swelling was performed bydispensing 1 mL DMF and incubating for 15 min (2) with 10 sshaking every minute. Fmoc deprotection was achieved by treatmentwith 40percent piperidine DMF for 3 min and 20percent piperidine inDMF for 12 min (10 s/min shaking). Peptide couplings were carriedout by double couplings with Fmoc-protected amino acids(5 equiv), HBTU (5 equiv), HOBt (5 equiv) and DIPEA (10 equiv) inDMF for 40 min (10 s/min shaking). At the respective position,Fmoc-F2Pmp-OH (3 equiv) was coupled in DMF (1 mL) by manualaddition using TBTU (3 equiv), HOBt (3 equiv) and DIPEA (6 equiv)for 3 h, after 3 min preactivation. In case of the sequences for which side-chain labeling with biotinor carboxyfluorescein was planned, an additional 4-methyltrityl-(Mtt-) protected lysine was coupled to the N-terminus. Toselectively remove the Mtt group the resin was washed for 1 minwith DCM (3), deprotection was then achieved by treatment with1.8percent TFA in DCM for 3 min (10). During the deprotection the DCMsolution turned yellow.For fluorescein-labeling of the amine side-chain 5(6)-carboxyfluorescein(3 equiv), HATU (3 equiv), HOAt (3 equiv) andDIPEA (6 equiv) were dissolved in DMF and pre-activated for3 min. The solution was aspirated and coupling was allowed toproceed for 1 h. This step was repeated 4 times.For biotin-labeling of the amine side-chain the resin waswashed for 1 min in NMP (3). D-(+)-Biotin (3 equiv), HATU(3 equiv), HOAt (3 equiv) and DIPEA (6 equiv) were dissolved inNMP and pre-activated for 3 min. The solution was aspirated andcoupling was allowed to proceed for 2 h. This step was repeated2 times. N-terminal acetylation (where applicable) was achieved by dispensing800 lL of a mixture of acetic anhydride/pyridine (1:9) andreaction twice for 5 min (10 s/min shaking). After each deprotection,coupling or acetylation step, 5 washings (1 min each) withDMF were performed (10 s/min shaking).After synthesis the resin was transferred in a 5 mL syringeequipped with a frit, washed with DCM for 1 min (3) and driedin high vacuum for at least 30 min. For cleavage 1 mL of a mixtureof TFA and TIS (20:1) was added. The syringe with the mixture waskept on a shaker for 3 h. Then the liquid phase was filtered into20 mL of ice-cold Et2O. Formed precipitate was centrifuged,washed with ice-cold Et2O (2 20 mL) and purified by HPLC. 4.1.2. Azide functionalization of the N-terminus; To the peptides with the longer carbon linker, 6-azidohexanoicacid was coupled (with standard coupling conditions) to the Nterminalamine.The N-terminal amine of the peptides with the shorter linkerwas converted to an azide functionality directly on solid support.Using the compound imidazole-1-sulfonyl-azide*HCl (synthesissee beneath) and modified conditions, which were reported forsolution phase chemistry from Goddard?Borger and Stick:8 Theresin was washed for 1 min each with DCM (2), DCM/MeOH(2) and MeOH (3). Then (for 40 mg resin, loading= 0.62 mmole/g) 1.4 equiv of imidazole-1-sulfonyl-azide*HClin 1 mL MeOH and 100 ll of a saturated and centrifuged solutionof CuSO4*5H2O was added. After 1 min, DIPEA (1.8 equiv) wasadded and the coupling was allowed to proceed for 1 h andrepeated once more with an intermediate washing with MeOH(3 1 min). |
- 7
-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
Ac-YLXKLLKLLXKLLK-NH2; X=propargyl glicine
[ No CAS ]
- 8
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
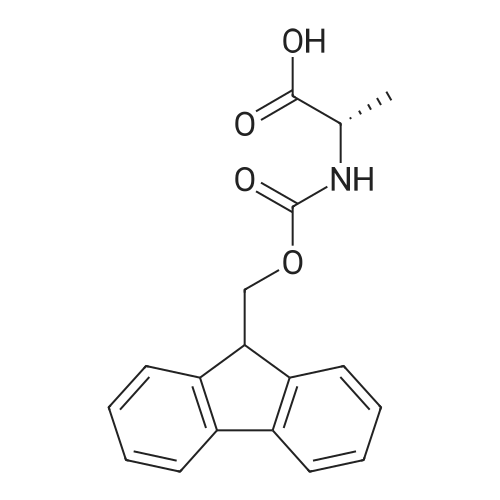 [ 35661-39-3 ]
[ 35661-39-3 ]

-
N4-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl)-N2-(9-fluorenylmethylcarbonyl)asparagine
[ No CAS ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
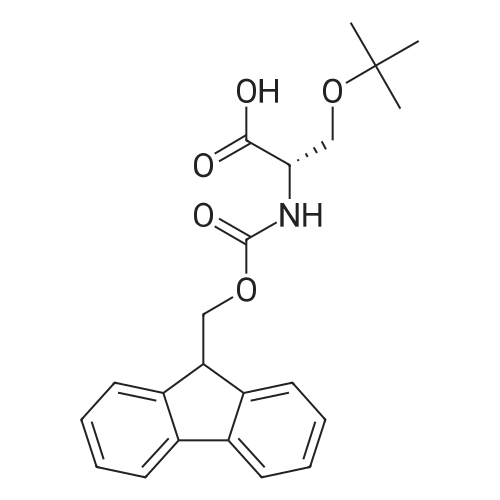 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
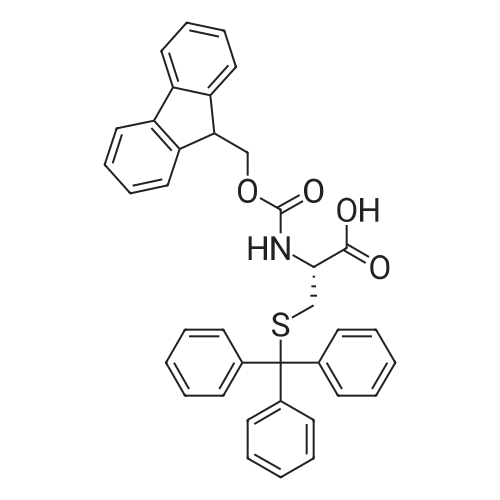 [ 103213-32-7 ]
[ 103213-32-7 ]

-
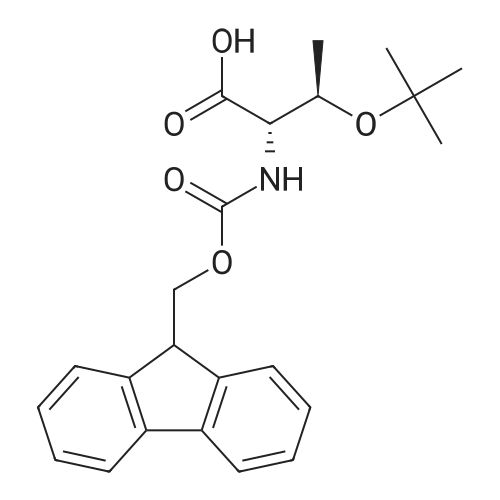 [ 71989-35-0 ]
[ 71989-35-0 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
 [ 109425-51-6 ]
[ 109425-51-6 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
KCNTATCATQRLANFLVHSS-(α-propargylglycinyl)-NFGPILPPTNVGS-(N4-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl)asparaginyl)-TY-NH2
[ No CAS ]
- 9
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 35661-39-3 ]
[ 35661-39-3 ]

-
N4-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl)-N2-(9-fluorenylmethylcarbonyl)asparagine
[ No CAS ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
 [ 103213-32-7 ]
[ 103213-32-7 ]

-
 [ 71989-35-0 ]
[ 71989-35-0 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
 [ 109425-51-6 ]
[ 109425-51-6 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
KCNTATCATQRLANFLVHSS-(N4-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl)asparaginyl)-NFGPILPPTNVGS-(α-propargylglycinyl)-TY-NH2
[ No CAS ]
- 10
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 35661-39-3 ]
[ 35661-39-3 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
 [ 103213-32-7 ]
[ 103213-32-7 ]

-
 [ 71989-35-0 ]
[ 71989-35-0 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
 [ 109425-51-6 ]
[ 109425-51-6 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
K-CNTATCATQRLANFLVHSSNNFGPILPPTNVGS-(α-propargylglycinyl)-TY-NH2
[ No CAS ]
- 11
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 35661-39-3 ]
[ 35661-39-3 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
 [ 103213-32-7 ]
[ 103213-32-7 ]

-
 [ 71989-35-0 ]
[ 71989-35-0 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
 [ 109425-51-6 ]
[ 109425-51-6 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
K-CNTATCATQRLANFLVHSS-(α-propargylglycinyl)-NFGPILPPTNVGS-(α-propargylglycinyl)-TY-NH2
[ No CAS ]
- 12
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 68858-20-8 ]
[ 68858-20-8 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 35661-39-3 ]
[ 35661-39-3 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
 [ 103213-32-7 ]
[ 103213-32-7 ]

-
 [ 71989-35-0 ]
[ 71989-35-0 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 132327-80-1 ]
[ 132327-80-1 ]

-
 [ 109425-51-6 ]
[ 109425-51-6 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
KCNTATCATQRLANFLVHSS-(α-propargylglycinyl)-NFGPILPPTNVGSNTY-NH2
[ No CAS ]
- 13
-
 [ 1266778-58-8 ]
[ 1266778-58-8 ]

-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
YGGFLRRIR-P(Di)-K-Pra-K-NH<SUB>2</SUB>; P(Di) = L-proline-4-spiro-3-(3H-diazirine)
[ No CAS ]
- 14
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
2-([(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-(4-benzoylphenyl)propanoic acid
[ No CAS ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
YGGFLRRIRPKLK-Bpa-Pra-NH<SUB>2</SUB>
[ No CAS ]
- 15
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
2-([(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-(4-benzoylphenyl)propanoic acid
[ No CAS ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
YGG-Bpa-LRRIRPKLK-Pra-NH<SUB>2</SUB>
[ No CAS ]
- 16
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
2-([(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-(4-benzoylphenyl)propanoic acid
[ No CAS ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
Pra-Bpa-KYRRIGPRGKL-NH<SUB>2 </SUB>
[ No CAS ]
- 17
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
 [ 35661-60-0 ]
[ 35661-60-0 ]

-
 [ 71989-31-6 ]
[ 71989-31-6 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 71989-26-9 ]
[ 71989-26-9 ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
Fmoc-Phe-(4-N3)-OH
[ No CAS ]

-
YGG-ArN<SUB>3</SUB>-LRRIRPKLK-NH<SUB>2</SUB>; ArN<SUB>3 </SUB>= 4-azidophenylalanine
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping