| 85% |
|
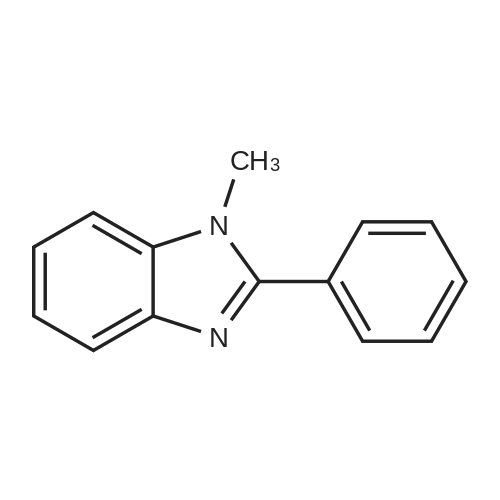
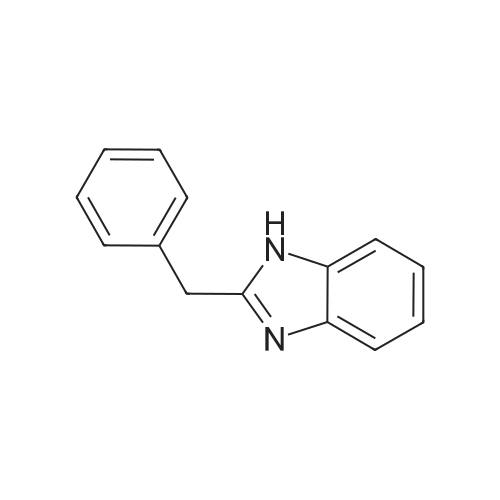
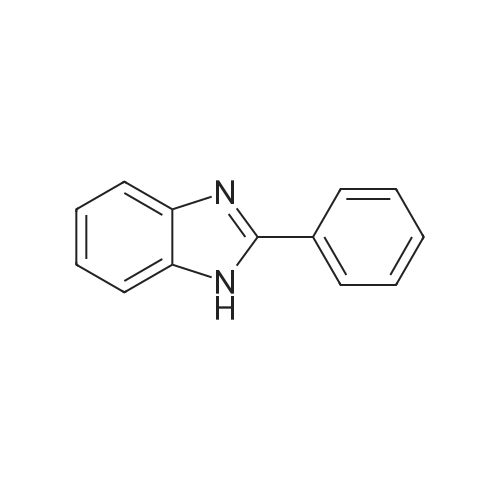
Powdered potassium hydroxide (2.24 g, 39.9 mmol) was added to a 250 mL round-bottom flask and vigorously stirred in DMSO (65 mL) for 30 min. A solution of 2- phenylbenzimidazole (4.11 g, 21.2 mmol) in DMSO (65 mL) was added to the basic DMSO solution and the mixture stirred for 45 min closed at room temperature. Iodomethane (1.4 mL, 22.5 mmol) was then added and the mixture stirred for 45 min. The mixture was then poured into a stirring solution of water (1.0 L) containing potassium hydroxide (5.0 g). Diethyl ether (300 mL) was then added and stirred until both layers were transparent. The organic layer was decanted and the same process was repeated with additional diethyl ether (2 x 200 mL). The combined organics were washed with water, brine, water, dried over magnesium sulfate, filtered, and evaporated at 44 °C under dynamic vacuum to yield 7 (3.74 g, 85percent) as a pale brown powder.1HNMR (500MHz, DMSO-d6, delta 7.86 (dd, J= 7.8, 1.7Hz, 2H), 7.69 (d, J= 7.9Hz,1H), 7.64-7.52 (m, 4H), 7.33-7.22 (m, 2H), 3.88 (s, 3H). 13C NMR (125 MHz, DMSOd 6, delta): 152.98, 142.47, 136.57, 130.15, 129.60, 129.28, 128.63, 122.32, 121.90, 118.98, 110.53, 31.64. |
| 62% |
|
Under an atmospheric nitrogen gas flow, sodium hydride in an amount of 12.8 mmol (containing 438 mg of 30 percent liquid paraffin) was placed into a three neck flask having a capacity of 100 milliliter, and adding n-pentane in an amount of 10 milliliter, the resultant solution was stirred at room temperature for 15 minutes. Subsequently, n-pentane was removed and then, after adding n-pentane again, the resultant solution was stirred. Repeating the above operation 4 times totally, the liquid paraffin was removed. Afterwards, the refined sodium hydride was vacume dried among the flask. Then, adding N,N-dimethylformamide in an amount of 15 milliliter and hpbi in an amount of 7.7 mmol (1.5 g), the resultant solution was stirred at a temperature of 35 °C for 1 hour and, further adding methyl iodide in an amount of 9.6 mmol (1.36 g), the resultant solution was further stirred at a temperature of 40 °C for 3 hours. After the resultant solution was cooled by leaving it standing, the solvent was removed by pressure reduction, and adding pure water, the resultant solution was stirred. Further, after adding dichloromethane, the resultant solution was divided by extraction. Gathering an organic layer, and after dehydration with the use of sodium sulfate, the solvent was concentrated by pressure reduction. A white precipitate generated by adding n-hexane was separated by filtration and 0.99 g of the aimed substance was obtained (yield: 62 percent). |
| 49% |
In acetone; at 20℃; for 6h; |
The synthesis of 1-methyl-2-phenyl-1H-benzoimidazole (Mpb) is accomplished by referring to methods disclosed in Popov, 1. I., Chem. Heterocycl. Compd. (EN), 1996, 32, 6, p.672-681. The synthetic method is outlined in Scheme 1. To 20 mL acetone was added 2-phenyl-1H-benzoimidazole (1.94 g, 10 mmol), followed by the injection of iodomethane (1.42 mL, 12 mmol). The mixture was stirred at room temperature for 6 h, sodium hydroxide solution was then added, and the mixture reacted for an additional 5 min. The reaction mixture was extracted with dichloromethane. The organic layer was dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography using n-hexanes/EA (v/v=80/20) as eluent. After the product was completely isolated, 1.03 mg (0.49 mmol) of the title compound was obtained (49percent yield). 1H NMR (CDCl3, delta): 3.87 (s, 3 H), 7.32-7.41 (m, 3 H), 7.51-7.56 (m, 3 H), 7.83-7.86 (m, 3 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping