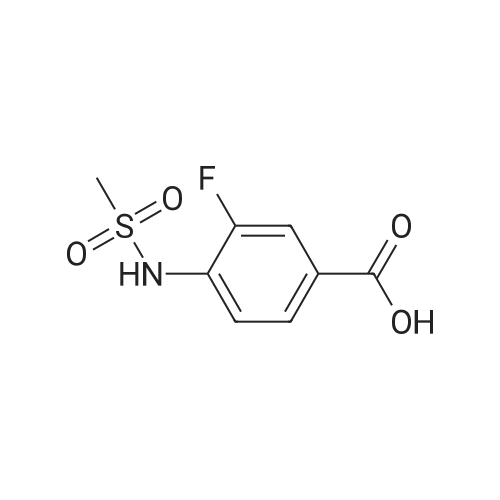
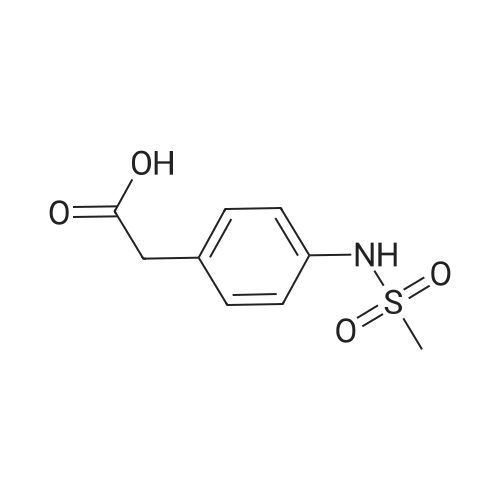
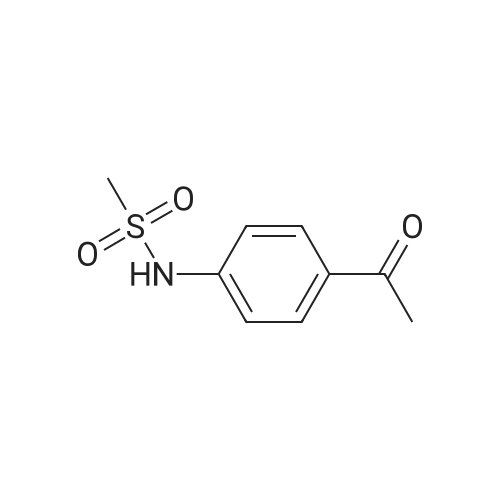
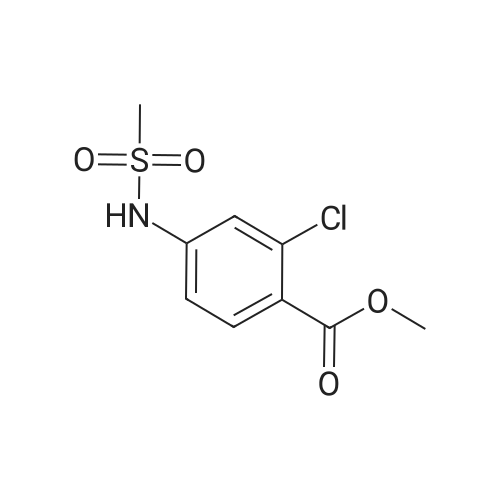
Alternatived Products of [ 7151-76-0 ]
Product Details of [ 7151-76-0 ]
| CAS No. : | 7151-76-0 |
MDL No. : | MFCD00025052 |
| Formula : |
C8H9NO4S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | SROHFTOYGFCJAF-UHFFFAOYSA-N |
| M.W : |
215.23
|
Pubchem ID : | 250653 |
| Synonyms : |
|
Application In Synthesis of [ 7151-76-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 7151-76-0 ]
- 1
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 126377-18-2 ]
[ 126377-18-2 ]

-
 [ 126377-23-9 ]
[ 126377-23-9 ]
- 2
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 137898-68-1 ]
[ 137898-68-1 ]

-
 [ 138490-68-3 ]
[ 138490-68-3 ]
- 3
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 138490-83-2 ]
[ 138490-83-2 ]

-
 [ 138490-76-3 ]
[ 138490-76-3 ]
- 4
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 138490-80-9 ]
[ 138490-80-9 ]

-
 [ 138490-64-9 ]
[ 138490-64-9 ]
- 5
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 658-78-6 ]
[ 658-78-6 ]

-
 [ 14001-51-5 ]
[ 14001-51-5 ]
- 6
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
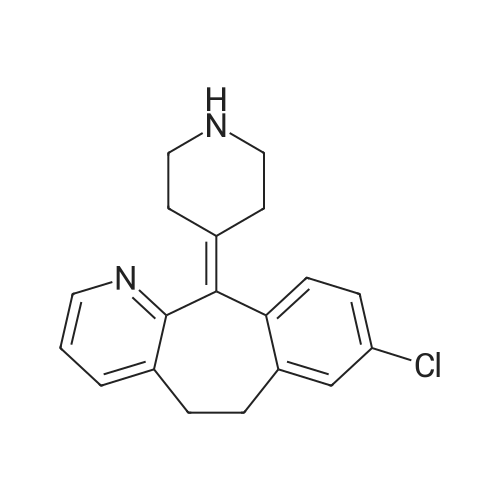 [ 100643-71-8 ]
[ 100643-71-8 ]

-
N-{4-[4-(8-Chloro-5,6-dihydro-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-piperidine-1-carbonyl]-phenyl}-methanesulfonamide
[ No CAS ]
- 7
-
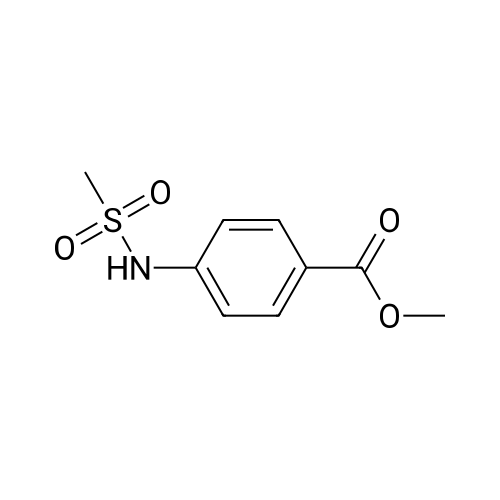 [ 50790-28-8 ]
[ 50790-28-8 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]
- 8
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
(±)-trans-3-amino-1-benzyloxycarbonyl-4-hydroxypyrrolidine
[ No CAS ]

-
(3R,4R)-3-Hydroxy-4-(4-methanesulfonylamino-benzoylamino)-pyrrolidine-1-carboxylic acid benzyl ester
[ No CAS ]

-
(3R,4R)-3-(4-Methanesulfonylmethyl-benzoylamino)-4-(4-methanesulfonylmethyl-benzoyloxy)-pyrrolidine-1-carboxylic acid benzyl ester
[ No CAS ]
- 11
-
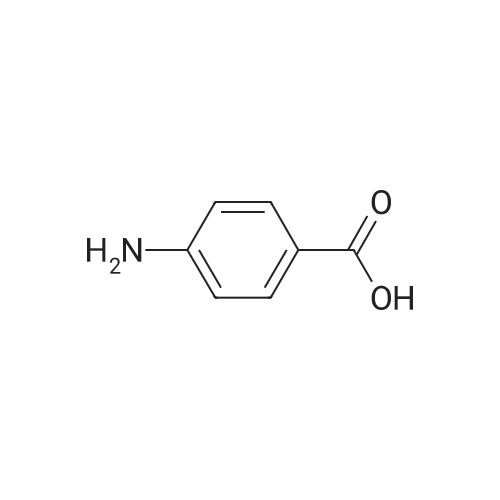 [ 150-13-0 ]
[ 150-13-0 ]

-
 [ 124-63-0 ]
[ 124-63-0 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Example 15 4-[1-Dodecanoyloxy-2-N-(4-carboxyphenyl)sulfonamido]ethyl-5-hydroxy-2(5H)-furanone (Compound 13) 4-Aminobenzoic acid is reacted with methanesulfonyl chloride (Compound 19) to give N-(4-carboxyphenyl)methanesulfonamide. |
- 12
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
trans-3-(4-methanesulfonamidobenzamido)-4-[4-(2-carboxy-6-hydroxybenzoyl)-3,5-dihydroxybenzoyloxy]pyrrolidine trifluoroacetate
[ No CAS ]
- 13
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
(3R,4R)-3-[3,5-Bis-benzyloxy-4-(2-benzyloxy-6-benzyloxycarbonyl-benzoyl)-benzoyloxy]-4-(4-methanesulfonylamino-benzoylamino)-pyrrolidine-1-carboxylic acid benzyl ester
[ No CAS ]
- 14
-
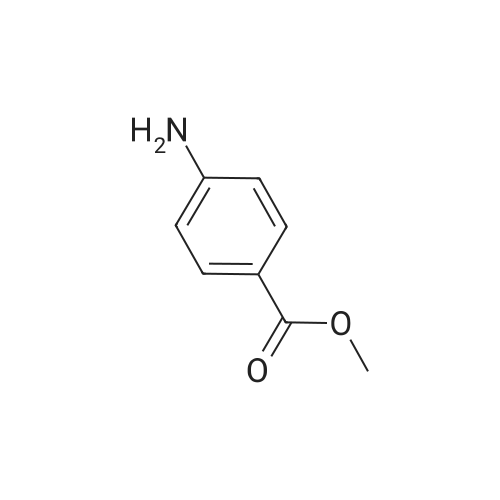 [ 619-45-4 ]
[ 619-45-4 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]
- 15
-
 [ 124-63-0 ]
[ 124-63-0 ]

-
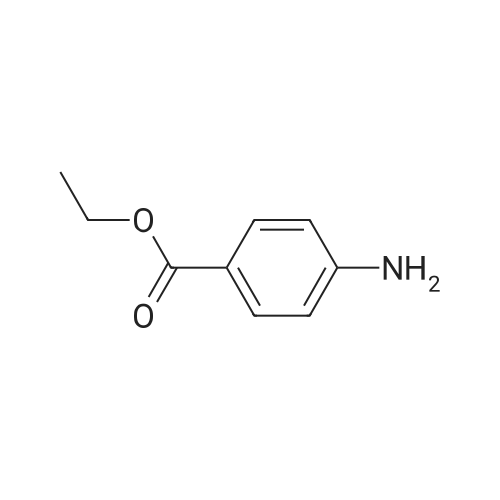 [ 94-09-7 ]
[ 94-09-7 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine; In methanol; dichloromethane; |
(a) 4-Methanesulfonylamino-benzoic acid. Ethyl 4-amino-benzoate (5 g, 30 mmol, 1.0 equiv) was mixed in 50 mL of CH2Cl2 with Et3N (6.1 g, 60 mmol, 2.0 equiv). The solution was cooled to 0 C. and methanesulfonyl chloride (3.8 g, 33 mmol. 1.1 equiv) was added as a solution in 15 mL of CH2Cl2 dropwise over a 15 min period. The reaction was stirred for 4 h at which time it was diluted with 50 mL of water. Layers were separated and the organic layer was washed with 50 mL of saturated sodium bicarbonate solution and concentrated. The resulting ester was dissolved in 50 mL of MeOH and treated with 50 mL of 5 N NaOH for 4 h. The reaction solution was extracted with Et2O and the aqueous layer was acidified to give a white precipitate that was collected by filtration. The solid was dried and used without further purification. |
|
With pyridine; In dichloromethane; |
Preparation 1 4-[(Methylsulfonyl)amino]benzoic acid To 215.2 g (2.72 mole) of pyridine in 1250 ml of dichloromethane is added 238 g (1.44 mole) of ethyl 4-aminobenzoate. After cooling to 0 C., a solution of 177.6 g (1.55 mole) of methanesulfonyl chloride in 250 ml of dichloromethane is added with stirring. Upon completion of the addition, the ice bath is removed and the reaction is stirred for one hour at room temperature. The reaction mixture is extracted with 3*500 ml of 4N sodium hydroxide and the aqueous extract is first washed with 3*500 ml of dichloromethane and then with 3*250 ml of ether. The aqueous extract is refluxed for two hours and allowed to cool overnight. The reaction is acidified with concentrated hydrochloric acid until a white precipitate forms. Collection by filtration followed by drying provides 310 g of the title compound. NMR (DMSO-d6): delta=3.2(s,3), 7.25-7.55(m,2) and 7.85-8.20 (m,2)ppm. |
- 16
-
 [ 148672-79-1 ]
[ 148672-79-1 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
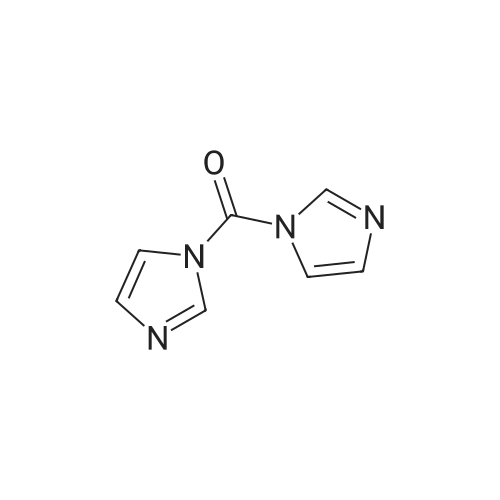 [ 530-62-1 ]
[ 530-62-1 ]

-
N-(1-Azabicyclo[3.3.1non-5-ylmethyl)-4-[(methylsulfonyl)aminol]benzamide
[ No CAS ]

-
sesquihydrate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 0.61 g (32%) |
In tetrahydrofuran; N-methyl-acetamide; methanol; acetonitrile; |
EXAMPLE 9 N-(1-Azabicyclo[3.3.1non-5-ylmethyl)-4-[(methylsulfonyl)aminol]benzamide A solution/suspension of 4-[(methanesulfonyl)amino]benzoic acid (1.08 g, 5.0 mmol) in anhydrous 1:1 tetrahydrofuran/dimethylformamide (4 mL) was treated with 1,1'-carbonyldiimidazole (0.89 g, 5.5 mmol), stirred for one hour, then degassed over 15 minutes under a stream of nitrogen. A solution of 1-azabicyclo[3.3.1]nonane-5-methanamine (0.85 g, 5.5 mmol) in anhydrous tetrahydrofuran (4 mL) was added, and the mixture was stirred at room temperature for 18 hours and at 50 C. for three hours, then concentrated in vacuo. The residue was triturated from acetonitrile/ether, then dissolved in 3.2 tetrahydrofuran/methanol and filtered through a short column of alumina (eluted with 3:2 tetrahydrofuran/methanol). The filtrate was concentrated in vacuo and recrystallized (2 crops) from 2-propanol/ether to afford 0.61 g (32%) of the sesquihydrate of the title compound as a voluminous colorless solid; mp 137-139 C. (foam). Analysis: Calc. for C17 H25 N3 O3 S.1.5H2 O: C, 53.95; H, 7.46; N, 11.10. Found: C, 54.14; H, 7.35; N, 10.49. |
- 17
-
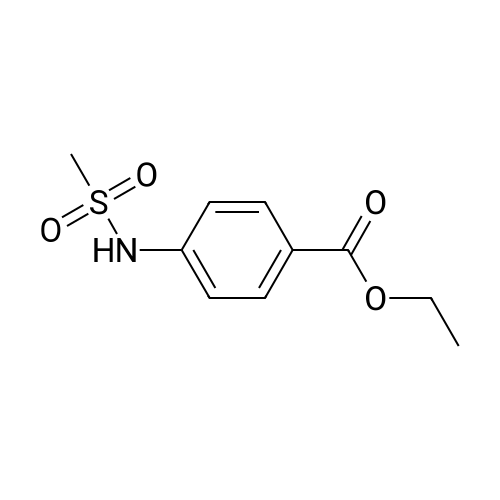 [ 7151-77-1 ]
[ 7151-77-1 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1.4 g (65%) |
In sodium hydroxide; |
EXAMPLE 16 STR20 4-[(Methylsulfonyl)amino]benzoic acid. A mixture of ethyl 4-[(methylsulfonyl)amino]benzoate (Lumma, W. C. et al. J. Med. Chem. 1987, 30, 758-763) (2.43 g, 0.01 mol) in 1N sodium hydroxide (22 mL, 0.022 mol) was stirred at room temperature for 24 h and then at 48 C. for 24 h. The mixture was acidified with concentrated hydrochloric acid to give a white precipitate which was separated by filtration. The cake was washed with water, dried, and recrystallized with ethanol-water to give 1.4 g (65%) of the title compound as white flakes, mp 248 C. Analysis: Calculated for C8 H9 NO4 S: C, 44.64; H, 4.21; N, 6.50 Found: C, 44.71; H, 4.26; N, 6.50 |
- 18
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
4-[(methylsulfonyl)amino]benzoic acid sodium salt
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydroxide; In water; |
Preparation 2 4-[(Methylsulfonyl)amino]benzoic acid sodium salt To 500 ml of water is added 63.4 g (1.59 mole) of sodium hydroxide and the mixture is stirred until all the solid has dissolved. To this is slowly added 310 g (1.44 mole) of <strong>[7151-76-0]4-[(methylsulfonyl)amino]benzoic acid</strong>. The reaction is stirred for 15 minutes at ambient temperature after all the solid has dissolved. After removal of the water in vacuo, the residue is triturated with isopropyl alcohol, evaporated, triturated with acetone and concentrated in vacuo. The solid is dried under vacuum for 72 hours to provide 333.7 g of the title compound. NMR (DMSO-d6): delta=3.0(s,3), 5.3-5.8(br s,2), 7.0-7.3 (m,2) and 7.8-8.1(m,2)ppm. |
- 19
-
 [ 7151-76-0 ]
[ 7151-76-0 ]

-
 [ 63421-72-7 ]
[ 63421-72-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With thionyl chloride; for 2h;Heating; |
A mixture of 2-chlorobenzoic acid (1b) (0.61 g, 3.92 mmol) and thionyl chloride (1 mL) was heated for 2 h. The excess of thionyl chloride was recovered under reduced pressure and the residue so obtained was dissolved in dry dioxane (1 mL). The solution was then dropwise added to mixture of 2-amino-4,5-dimethoxybenzamide (2) (0.7 g, 3.57 mmol) and TEA (1.2 mL, 8.92 mmol) in dry dioxane (2 mL) at 10 C. The reaction mixture was stirred at rt for another 1 h and quenched into cold water (50 mL). The precipitate formed was filtered, washed with water and dried to obtain compound 3b (0.85 g, 71%): mp 213-215 C (dec.); IR (KBr,): 3406, 3305, 1658, 1645, 1612, 1525, 1253, 1078 and 1039 cm-1. |
- 20
-
 [ 619-45-4 ]
[ 619-45-4 ]

-
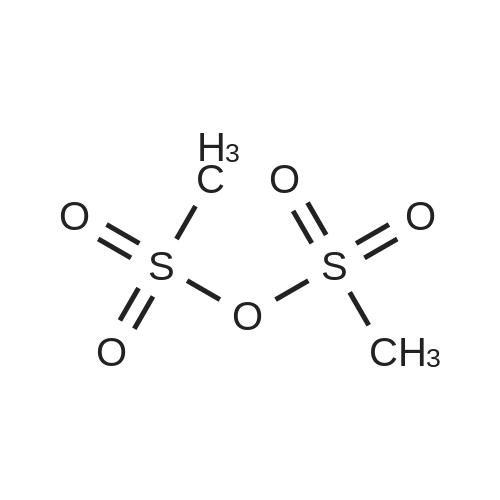 [ 7143-01-3 ]
[ 7143-01-3 ]

-
 [ 7151-76-0 ]
[ 7151-76-0 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping