| 68% |
|
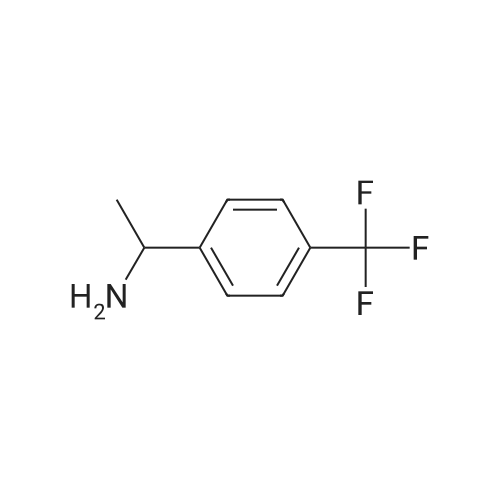
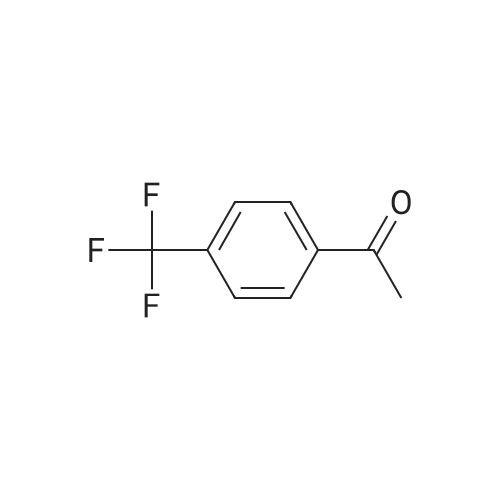
Ti(OiPr)4 (31.5 mL, 106 mmol) was added to (p-trifluoromethyl)acetophenone 108 (10.0 g, 53.1 mmol) in NH3 (2.0 M in EtOH, 133 mL) and the resultant mixture was stirred at rt for 6 h. NaBH4 (3.01 g, 79.7 mmol) was added at 0 C, and the resultant suspension was stirred at rt for 3 h, then poured into 2.0 M aq NH4OH (200 mL). The resultant suspension was filtered (eluent EtOAc) and the aqueous layer was extracted with EtOAc (2*80 mL). The combined organic extracts were extracted with 1.0 M aq HCl (3*50 mL) and the combined aqueous extracts were washed with EtOAc (3*30 mL), treated with 2.0 M aq NaOH until pH>8 was observed, then extracted with EtOAc (4*50 mL). The combined organic extracts were washed with brine (200 mL), then dried and concentrated in vacuo to give (RS)-117 as a pale yellow oil (6.84 g, 68%);43 δH (400 MHz, CDCl3) 1.40 (3H, d, J 6.6, C(α)Me), 1.59 (2H, br s, NH2), 4.20 (1H, q, J 6.6, C(α)H), 7.48 (2H, d, J 7.8, C(2)H, C(6)H), 7.59 (2H, d, J 7.8, C(3)H, C(5)H). |
| 52% |
|
A mixture of 1-(4-(trifluoromethyl)phenyl)ethanone (400 mg, 2.13 mmol), Ti(O-i-Pr)4 (1.25 mL, ~4.25 mmol) and ammonia in EtOH (2 M, 5.30 mL, ~10.6 mmol) was stirred under argon at room temperature for 24 h NaBH4 (120 mg, 3.19 mmol) was then added, and the resulting mixture was stirred for another 24 h. The pH of the reaction mixture was adjusted to pH 2 using HCl (6 M), and washed with tert-butyl methyl ether (TBME) (3 × 20 mL). Using NaOH (pellets) the pH was adjusted to ca 10, and the mixture was extracted with TBME (6 × 30 mL). The combined organic phase was dried over MgSO4, and the solvent was removed under reduced pressure to give 210 mg (1.11 mmol, 52%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.58 (m, 2H), 7.46 (m, 2H), 4.19 (q, J = 6.7, 1H), 1.38 (d, J = 6.7, 3H), 1.51 (s, 2H, NH2). 13C NMR (100 MHz, CDCl3) δ: 152.1 (q, J = 1.1), 129.4 (q, J = 31.5), 126.5 (2C), 125.8 (q, J = 3.8, 2C), 124.6 (q, J = 270.9), 51.4, 25.1. |
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; ammonium formate; In methanol; at 70℃; for 7h;Inert atmosphere; |
General procedure: The corresponding ketone (20 mmol, 1.0 eq) and CH3OH (20 mL) were added to a 250 mL Schlenk tube containing [RhCp*Cl2]2 (61.8 mg, 100 μmol, 0.005 equiv) and HCOONH4 (6.36 g, 100 mmol, 5.0eq). The brown mixture was frozen, and the whole system was evacuated. The system was closed and then stirred at 70 C for 7 h. After the dark green resulting solution was cooled to room temperature, 1M aqueous HCl solution (38.4 mL) was added, and the mixture was washed twice with CH2Cl2 (5 mL) to remove the neutral compounds. After addition of a cold 12 M aqueous NaOH solution (3.6 mL) to the aqueous layer, the mixture was extracted six times with CH2Cl2 (12 mL). The combined organic layers were dried over anhydrous Na2SO4. Filtration and evaporation under reduced pressure gave crude amine,which was used without purification. All the crude corresponding amine was dissolved in dichloromethane (50 mL), and TCCA (trichloroisocyanuric acid) (3.2 g, 14 mmol) was added in a250 ml round-bottom flask at 0 C. Then, the mixture was stirred at ambient temperature during 1 h. Triethylamine (6.0 g, 6 mol) dissolved in dichloromethane (50 mL) was added, and the resulting mixture was washed with water (200 mL) and hydrochloric acid (1 M, 200 mL)successively. The organic layer was dried over anhydrous sodium sulfate. After concentration under reduced pressure, purification by column chromatography on silica gel (n-hexane/EtOAc:40/1) afforded pure product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping