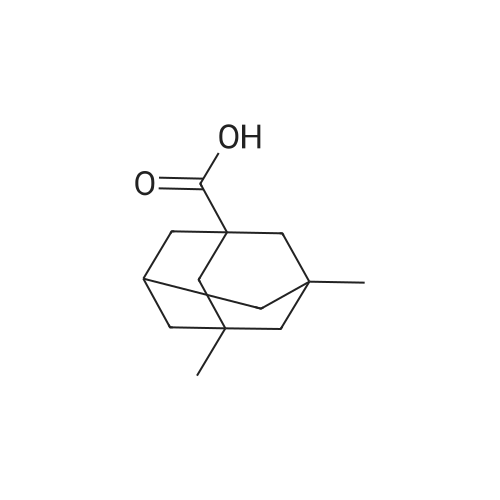
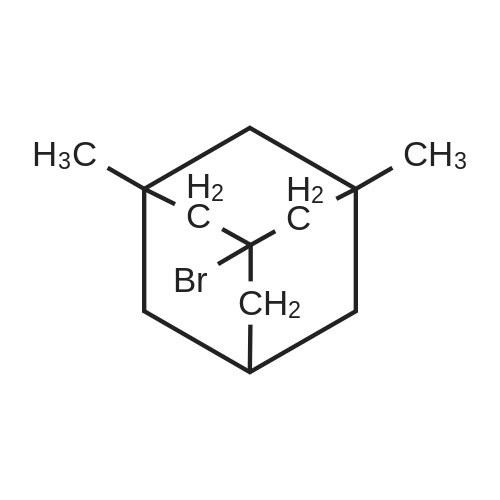
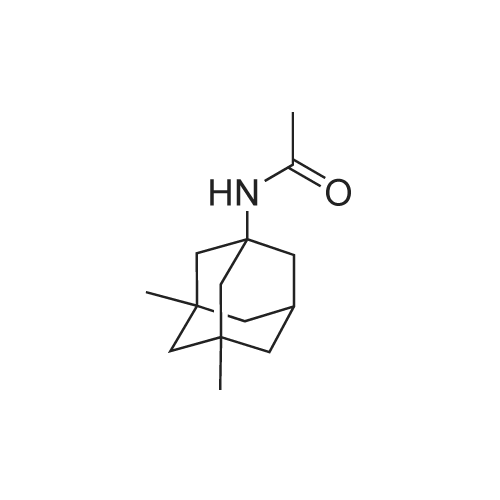
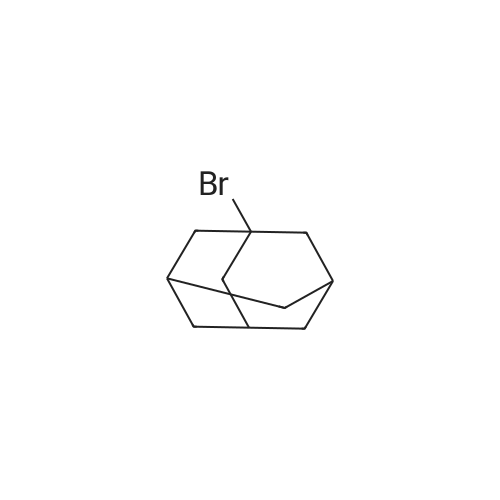
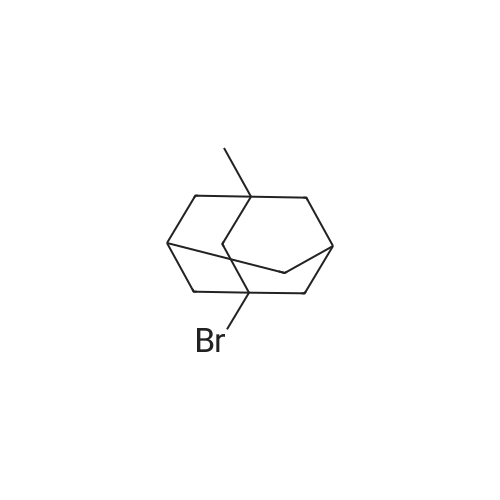
|
With bromine; at 25 - 51℃; for 12.5h;Product distribution / selectivity; |
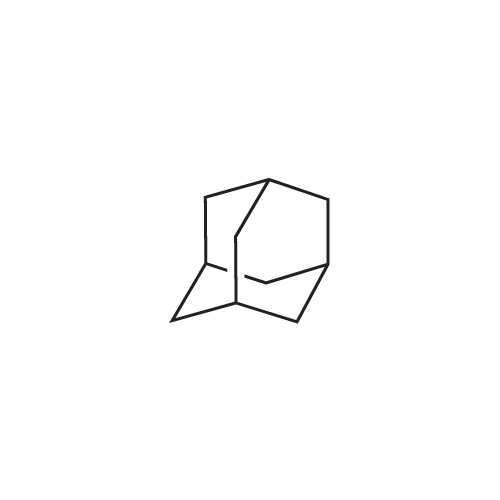
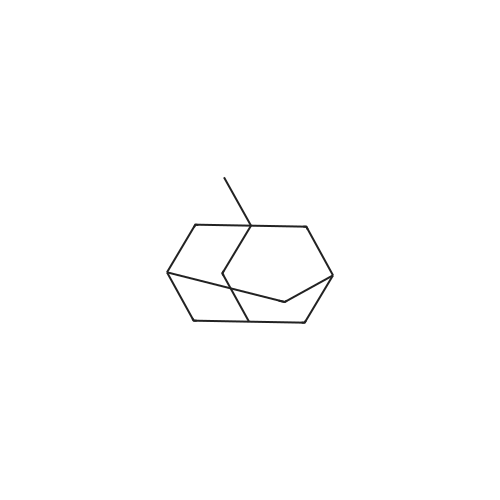
1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (67.36 mL, 4.5 equivalent) at 25-300C within 30 minutes. The reaction mixture was heated at 48-51 C and maintained for 12 Hrs. After heating for 12 Hrs, the reaction mixture was cooled to 10-150C and then methylene chloride (800 mL) was added at 5-100C. Reaction mixture was stirred at 5-100C for 30 minutes. To the reaction mixture water (1.5 L) was poured at 5-10 C within 1 Hr and stirred for 30 minutes. Sodium metabisulfte (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-100C . After stirring the layers were settled for 30 minutes and then separated out. To the organic (MDC) layer solution of 1% sodium bicarbonate (500 mL) was added at 5-100C and stirred for 30 minutes and after stirring layers were settled and separated out. Further to the organic layer (MDC) Brine solution (500 mL) was added and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. Organic layer (MDC) was dried over anhydrous sodium sulfate and wash the bed with MDC (48 mL). Further MDC layer was distilled atmospherically below 600C and further traces of MDC was removed under vacuum for 1 Hr below 40 C to afford the title compound l-bromo-3,5-dimethyl adamantane.; Example 1 (b) : Preparation of l-Bromo-3,5-dimethyl adamantane with 6.5 equivalent of bromine 1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (97.36 mL, 6.5 equivalent) at 25-300C within 30 minutes. The reaction mixture was heated at 48-510C and maintained for 12 Hrs. After heating for 12 Hrs, the reaction mixture was cooled to 20-250C and then methylene chloride (800 mL) was added at 5-100C. Reaction mixture was stirred at 5-100C for 30 minutes. The <n="14"/>reaction mixture was poured into water (1.5 L) at 5-100C within 1 Hr and stirred for 30 minutes. Sodium metabisulfite (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. To the organic (MDC) layer solution of 1% sodium bicarbonate (500 mL) was added at 5-100C and stirred for 30 minutes and after stirring layers were settled and separated out. Further to the organic layer (MDC) Brine solution (500 mL) was added and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. Organic layer (MDC) was dried over anhydrous sodium sulfate and wash the bed with MDC (48 mL). Further MDC layer was distilled atmospherically below 600C and further traces of MDC was removed under vacuum for 1 Hr below 400C to afford the title compound l-bromo-3, 5 -dimethyl adamantane. |
|
With bromine; at 20 - 80℃; for 14h;Inert atmosphere;Product distribution / selectivity; |
Example 1[007O] A clean enameled reactor is inerted by purging 3 times with nitrogen. Temperature in the reactor is adjusted to 2O0C. 1.22 kmol of 1 ,3- dimethyladamantan is introduced into the reactor under nitrogen atmosphere. Consequently, 3.66 kmol of bromine is introduced into the reactor under constant agitation at 90 rpm. The reaction mass is heated up to 8O0C in approximately 8 hours under constant agitation at 90 rpm. The reaction mass is agitated for another 6 hours at 80C.105577P502PC [0071] A second reactor is prepared and the atmosphere in the reactor is inerted by purging the reactor 3 times with nitrogen. 7.9 kmol of formamide is introduced under nitrogen atmosphere at 22C into the reactor. Consequently, formamide is heated up to 75C under constant agitation at 100 rpm. The reaction mass in the first reactor is slowly, over a time period of 4 hours, transferred into the reactor containing formamide. The resulting mixture is agitated for 2 hours. After the preceding of 2 hours of agitation, the reactor is immediately cooled to 120C. Methylene chloride is introduced to this reactor at 12C. Immediately thereafter, bisulfite solution is prepared in a third reactor at 200C and consequently cooled down to 1O0C. Slowly, over a time period of 2-3 hours, the bisulfite solution is transferred under constant agitation at 100 rpm into the second reactor containing the reaction mass. After 15 minutes two phases are formed; the brown upper phase containing the product and the aqueous layer phase containing the salts. The two phases are separated. Separately, a sodium bicarbonate solution is prepared and introduced into the brown organic phase. After 30 minutes, the mass is rinsed with Water for Injection. 7.06 kmol of hydrochloric acid is introduced to the resulting reaction mass and is heated to until reflux (T=98- 108C). The resulting slurry is cooled immediately to 80C within 2.5 hours. The output is memantine HCI. |
|
With bromine;hydrogen bromide; acetic acid; at 25 - 55℃; |
Example-1: Preparation of l-bromo-3,5-dimethyl adamantane[71] Charge <strong>[702-79-4]1,3-dimethyl adamantane</strong> (lOOgm. 0.6 moles) at 25-300C. Charge HBr inAcOH (1 ml) at 25-300C. Heat reaction mixture up to 50-550C. Add drop- wise bromine (124.7 ml, 2.41 moles) slowly at 50-550C. Maintain it for 12 hours at 50-550C. Distil out excess bromine atmospherically up to 85C. Cool down the reaction mixture to 25-300C. Add MDC (800 ml) into it. Stir for 30 minutes at 25-300C. Cool MDC reaction mixture to 5C. Added drop wise previously prepared 1500 ml 5% solution of sodium hydrosulfite in DM Water into reaction mixture. Separate the MDC layer and discard aqueous layer. Wash it twice with DM water (100 ml). Distil out MDC completely atmospherically up to 55C. Remove the traces of MDC under vacuum (50 - 100 mm) at 50-650C. Oily residue is obtained.[72] Results :-[73] Dry wt:- 140.0 g[74] Yield (%w/w):- 1.4[75] Purity:- 99.7% |
|
With bromine; for 16h;Reflux; |
Example 3Preparation of 1-bromo-3,5-dimethyladamantane[0064] 1 , 3-dimethyladamantane containing 0.05% or less of the impurity 1 ,3,5, trimethyladamantane, as prepared in Example 2, is treated with bromine (3 equivalents) and heated to reflux for 16 h. The reaction mixture is cooled to about 15 0C and quenched with sodium bisulphite in methylene chloride. The aqueous layer is removed, and the organic layer is washed with water. The organic layer is concentrated in vacuo to yield 1-bromo-3,5-dimethyladamantane as an oil. |
|
With bromine; at 28℃; for 26.5h; |
EXAMPLE 1; PREPARATION OF 1-BROMO-3.5-DIMETHYL ADAMANTANE (FORMULA III); 8.0 liters of bromine was taken into a reactor and 5.1 kg of 1 ,3-dimethyl adamantane was added into the reactor slowly at 28 C in 2.5 hours. The reaction mass was maintained at 28 C for 24 hours. Reaction completion was checked using a gas chromatographic technique. After the reaction was completed, the bromine was distilled off from the reaction mass at a temperature of below 40 C. After the completion of distillation, 13.4 kg of the title compound in the form of a residue was recovered from the reactor.Purity by GC: 98.35%,1 ,3-dimethyl adamantane: 0.53%.1-hydroxy-3,5-di methyl adamantane: 0.66%. |
|
With bromine; at 30℃; for 24h;Product distribution / selectivity; |
EXAMPLE 7; PREPARATION OF 1-N-FORMYL-3.5-DIMETHYL ADAMANTANE (FORMULA VI); 13 liters of bromine was taken into a reactor and 8.3 kg of <strong>[702-79-4]1,3-dimethyl adamantane</strong> was added to it at 30 C. The reaction mass was maintained at 30 C for 24 hours. Reaction completion was checked using a gas chromatographic technique. 200 liters of formamide was taken in another reactor and the reaction mass from the previous reactor was added to it. The previous reactor was rinsed with 8 liters of formamide and added to the reaction mass. The temperature of the reaction mass was raised to 155 C. The reaction mass was maintained at 155 C for 8 hours. Reaction completion was checked using gas chromatography. After the reaction was completed, the reaction mass was cooled to 4 C and maintained for 1.5 hours. 125 liters of chilled water was added to the reaction mass at 4 C. 125 liters of dichloromethane was added to the reaction mass and the EPO <DP n="16"/>temperature was raised to 25 C. The reaction mass was then filtered over a celite bed and the bed was washed with 42 liters of dichloromethane. The filtrate was allowed to settle and the organic layer was separated. The aqueous layer was extracted with 124.5 liters of dichloromethane in two equal lots. The combined dichloromethane layer was washed with 166 liters of 10% aqueous solution of sodium bicarbonate in two equal lots. The dichloromethane layer was dried over sodium sulfate and distilled atmospherically to dryness at 40 C to yield 18.2 kg of the title compound.Purity by GC: 96.29% 1 ,3-dimethyl adamantane: less than 0.004%.1-hydroxy-3,5-dimethyl adamantane: 2.81 %.1-bromo-3,5-dimethyl adamantane: 0.05%. |
|
|
100 gm of <strong>[702-79-4]1,3-Dimethyladamantane</strong> was talcen into a 500 ml 4-necked RBF at room temperature (25-35C). 403 gm of bromine was added at 25-35C over 30-120 minutes and stirred for 10-20 minutes at room temperature .The temperature was raised to reflux (Temp: 60-660C.) and maintained at reflux for about 6 hrs. The reaction was monitored for completion. The reaction mass was cooled and excess bromine was distilled off completely under vacuum to get residue. The residue was cooled to room temperature (25-35C). Dichloromethane (400 ml) was added and stirred for 5-15 minutes to make clear solution. 10% Sodium metabisulphite solution (500ml) was added at RT over 15-60 min. Stirred for 30 minutes and separated the layers. With usual workup organic layer was separated. The organic layer was dried over anhydrous sodium sulphate, filtered and washed the bed with dichloromethane (50ml). Carbon treatment was given with usual workup and dichloromethane was distilled off completely to get l-Bromo-3,5-dimethyladamantane weight: 135 grams Purity by GC: 99.1% |
| 120 g |
With N-Bromosuccinimide; In dichloromethane; at 23℃; for 24h; |
In a 5L reaction flask, 164g of dimethyl adamantane were added in sequence, 200g NBS and 2L of dichloromethane, React at room temperature for 24 h, Filter, filter cake washed to neutral, Drying and recrystallization of ethanol gave 120 g of pale yellow crystals. |
| 125 g |
With N-Bromosuccinimide; In dichloromethane; at 20℃; for 24h; |
In the reaction flask, 164g of <strong>[702-79-4]1,3-dimethyl-adamantane</strong>, 200g of NBS and 2L of dichloromethane were sequentially added, and reacted at room temperature for 24 hours, filtered, and the filter cake was washed with water until neutral, and dried.The ethanol was recrystallized to give a pale yellow crystal of 125 g. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +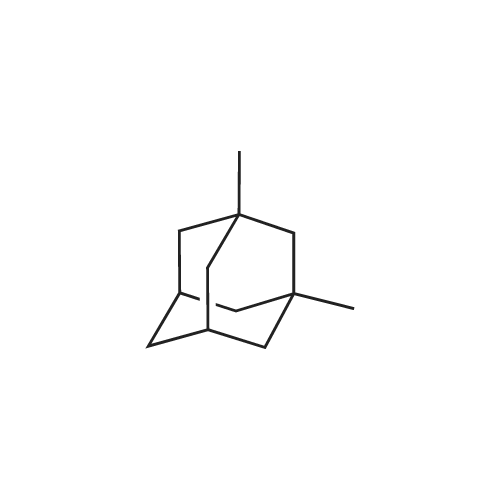

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping