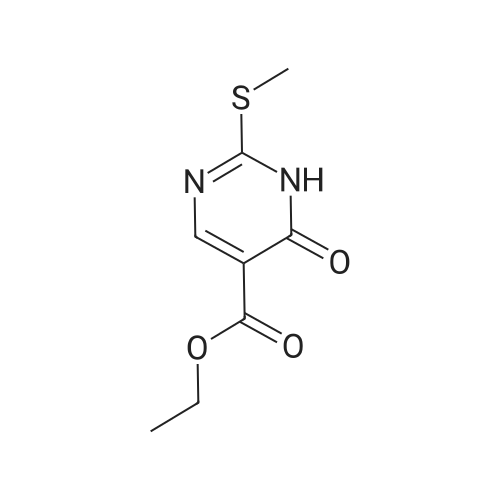
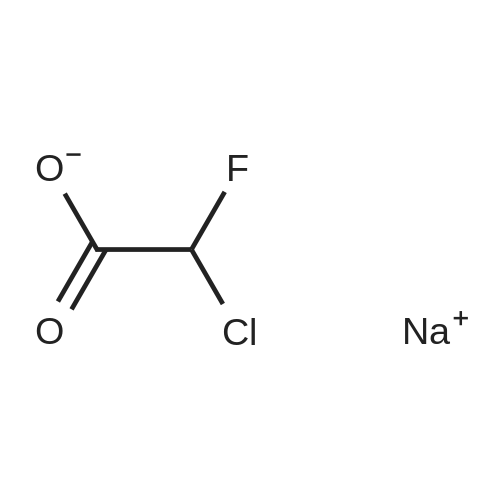
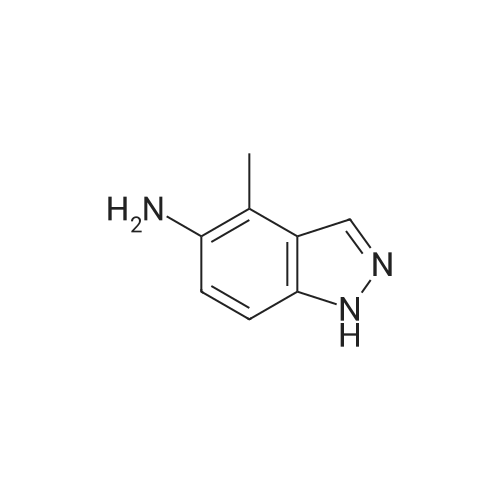
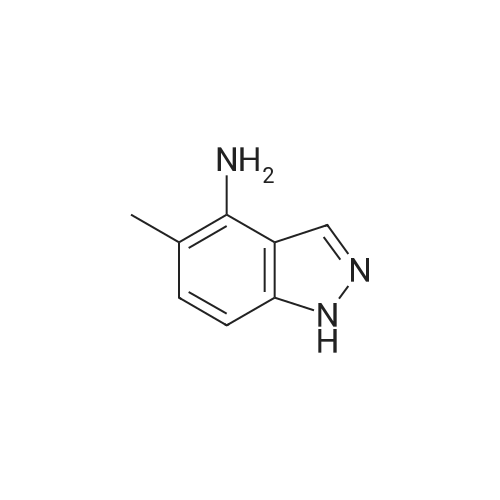
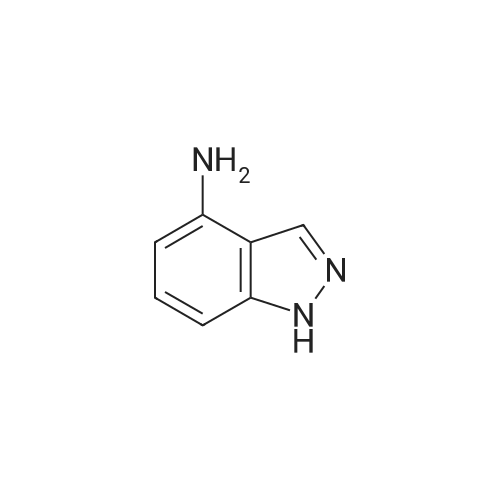
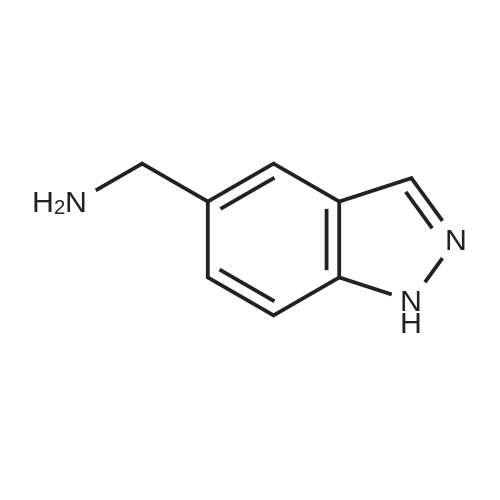
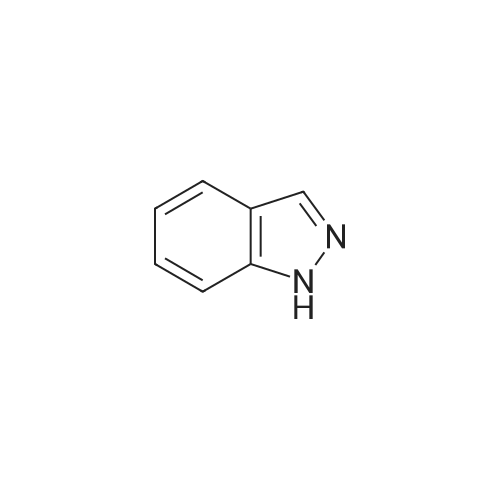
| 80% |
|
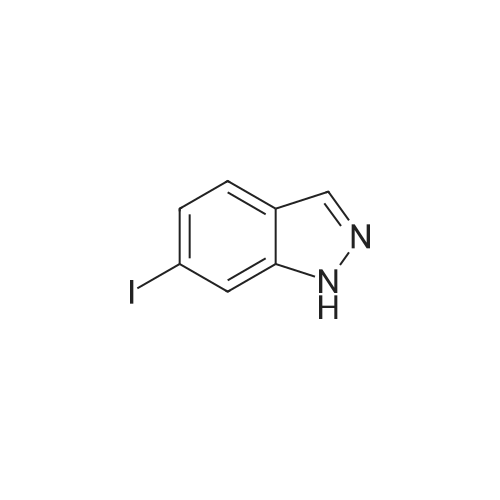
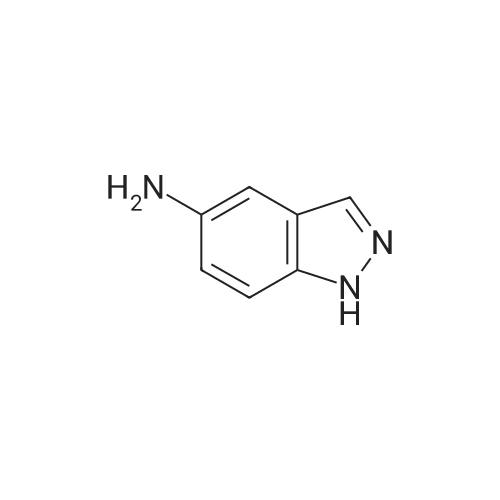
A concentrated hydrochloric acid (35 mL, 420 mmol) and an aqueous solution (30 mL) of sodium nitrite (6.64g, 96 mmol) were added to a suspension prepared by adding water (30 mL) to 6-aminoindazole (10.4 g, 78 mmol) at0C and stirred at 0C for 30 minutes. Subsequently, to this solution, an aqueous solution (30 mL) of potassium iodide(15.91 g, 96 mmol) was added at 0C, stirred at room temperature for 30 minutes, to which dichloromethane (80 mL)was then added, and stirred at 40C for 2 hours. The reaction mixture was cooled down to 0C, then adjusted to pH =14 with a 3N sodium hydroxide aqueous solution, and the precipitate was taken by filtration. The resulting precipitatewas washed with 10% sodium thiosulfate, dissolved in tetrahydrofuran, and then silica gel was added. After stirring atroom temperature for 1 hour, hexane (600 mL) was added and filtered. The residue was washed twice with a THF/hexane(1/3 (v/v)) solution, then the solvent was distilled away under a reduced pressure to obtain the title compound (15.23 g,80%) as an orange powder.1H NMR (400 MHz, CDCl3) δ 10.24 (1H, br, s), 8.04 (1H, br s), 7.92 (1H, br s), 7.51 (1H, br d, J = 8.4 Hz), 7.46 (1H, dd,J = 8.4, 1.2 Hz). |
| 48% |
With sodium hydroxide; concentrated aqueous HCl; Ki; sodium hydrogencarbonate; sodium nitrite; In tetrahydrofuran; hexane; water; |
(i) To 6-aminoindazole (40.8 g, 0.3065 mol, 1 equiv) in a 2-liter (2-L) round-bottom flask containing a large magnetic stir bar was added ice (256 g), followed by water (128 mL) and the reaction vessel was lowered into an ice bath. To this stirring slurry at 0 C. was added concentrated aqueous HCl (128 mL, 1.53 mol, 5 equiv). Immediately after, a solution of NaNO2 (23.3 g, 0.338 mol, 1.1 equiv) in water (96 mL) was added. After 10 min of stirring at 0 C., KI (61 g, 0.368 mol, 1.2 equiv) was added very slowly at first (~100 mg at a time because the first small bits of KI cause an abrupt evolution of gas) then more rapidly (5 min total time). The cold bath was removed and the reaction mixture was warmed to 40 C. (gas evolved). When the rate of gas evolution decreased (~30 min) the reaction mixture was warmed to 50 C. for 30 min. The mix was then cooled to 23 C., and 3N NaOH (320 mL) was added to neutralize followed by 50% saturated NaHCO3 (320 mL). The slurry was then filtered through a Buchner funnel to give a dark reddish-brown solid. The solid was taken up in warm THF (800 mL) and silica (600 mL dry) was added with stirring. To this slurry was added hexane (1.2 L) and the mix was vacuum filtered through a pad of silica (300 mL) in a large fritted filter. The silica was further washed with 2 L of 40% THF in hexane. The filtrates were combined and concentrated under reduced pressure to give a solid. The solid was further triturated with ethyl acetate (~100 mL), filtered and dried under reduced pressure to give 6-iodo-1H-indazole as a light brown solid (36.1 g, 48% yield): Rf sm 0.12, p 0.48 (Hex-EtOAc 1:1); 1H NMR (300 MHz, CDCl3) 7.9 (s, 1H), 7.8 (s, 1H), 7.42 (d, 1H), 7.33 (d, 1H); MS (ES) [m+H]/z Calc'd 245, Found 245, [m-H]/z Calc'd 243, Found 243. |
|
|
Example 12 6-Iodo-1H-indazole (compound 20) sodium nitrite (5.87 g, 85 mmol) in water (20 ML) was added dropwise to an ice-cooled solution of 6-aminoindazole (10 g, 75.6 mmol) in DMF (80 ML) and hydrochloric acid (6M, 40 ML).. The mixture was stirred for 30 minutes.. potassium iodide (13.5 g) was then added in small portions (gas evolution occurred) and the mixture stirred for 1 h before warming to room temperature for 16 h.. The reaction was neutralized with aqueous sodium bisulfite, followed by aqueous sodium hydroxide.. The mixture was filtered to remove solids, and the solid was washed with water to remove impurities, and then with ethyl acetate and THF to collect the product.. The organic washes were evaporated and recombined with the aqueous layer for extraction with ethyl acetate (3*250 ML).. The organic layer was washed sequentially with water and brine, dried over sodium sulfate, and the solvent removed in vacuo.. Filtration chromatography on silica gel (35-60% ethyl acetate in hexane) gave a yellow solid which was triturated firstly with 50% ethyl acetate in hexane and then with ethyl acetate to yield the product (4.96 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping