| 60% |
|
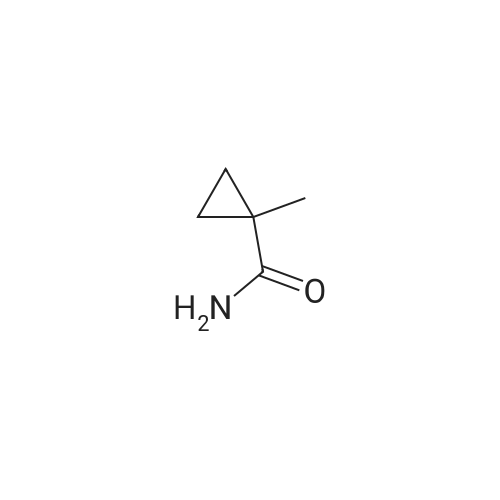
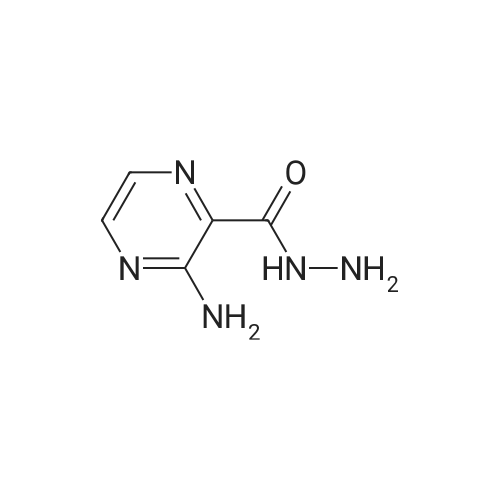
Hydrazine hydrate (34 mL, 1.1 mol) was added portionwise to a stirred suspension of methyl 3-aminopyrazine-2-carboxylate 37 (21.3 g, 139 mmol) in ethanol (65 mL) at room temperature. The resulting slurry was stirred at 60 C for 2 h, cooled to room temperature, and filtered. The solid was washed with cold ethanol (2 x 25 mL) and dried to a constant weight to afford <strong>[6761-52-0]3-aminopyrazine-2-carbohydrazide</strong> (20.75 g, 97%) as a beige solid. TBTU (5.77 g, 18.0 mmol) was added portionwise over 15 min to a stirred suspension of DIPEA (8.5 mL, 49 mmol), 1-methylcyclopropanecarboxylic acid (1.63 g, 16.3 mmol) and <strong>[6761-52-0]3-aminopyrazine-2-carbohydrazide</strong> (2.50 g, 16.3 mmol) in acetonitrile (40 mL). The mixture was stirred at 80 C for 20 min, and then cooled to 0 C. DIPEA (8.5 mL, 49 mmol) followed by 4-methylbenzene-1-sulfonylchloride (9.34 g, 49 mmol) were added over a period of 15 min. The reaction mixture was brought to reflux and allowed to stir at room temperature for 14 h. The mixture was concentrated, and the residue was diluted with DCM, washed with water and brine, dried over magnesium sulfate and concentrated. The crude product was purified by flash chromatography on silica gel eluting with 0 to 40% ethyl acetate in dichloromethane. After evaporation of the solvent, the resulting solid was triturated with diethyl ether, filtered, washed with the minimum of diethyl ether and dried to afford 3-(5-(1-methylcyclopropyl)-1,3,4-oxadiazol-2-yl)pyrazin-2-amine (2.12 g, 60%). NBS (1.87 g, 10.5 mmol) was added portionwise to a solution of 3-(5-(1-methylcyclopropyl)-1,3,4-oxadiazol-2-yl)pyrazin-2-amine (2.08 g, 9.6 mmol) in THF (30 mL). The reaction mixture was stirred at room temperature for 16 h and concentrated. The residue was dissolved in dichloromethane (150 mL), washed with water (2 x 40 mL), brine, dried over magnesium sulfate and concentrated. After evaporation of the solvents, the crude product was purified by flash chromatography on silica gel eluting with 0 to 10% ethyl acetate in dichloromethane. The solvent was evaporated to dryness to afford 5-bromo-3-(5-(1-methylcyclopropyl)-1,3,4-oxadiazol-2-yl)pyrazin-2-amine (2.50 g, 88%) as a yellow solid. Bis(triphenylphosphine)palladium(II) chloride (59 mg, 0.08 mmol) was added in one portion to a stirred solution of 5-bromo-3-(5-(1-methylcyclopropyl)-1,3,4-oxadiazol-2-yl)pyrazin-2-amine (500 mg, 1.69 mmol) and hexamethyldistannane (0.49 mL, 2.4 mmol) dissolved in dioxane (8 mL) under argon. The resulting suspension was stirred at 80 C for 1 h. The mixture was evaporated. The crude product was purified by flash chromatography on silica gel eluting with 10 to 30% ethyl acetate in dichloromethane. The solvent was evaporated to dryness to afford 3-(5-(1-methylcyclopropyl)-1,3,4-oxadiazol-2-yl)-5-(trimethylstannyl)pyrazin-2-amine 38a (314 mg, 49%) as a yellow crystalline solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping