| 74% |
With potassium carbonate;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran;Inert atmosphere; Reflux; |
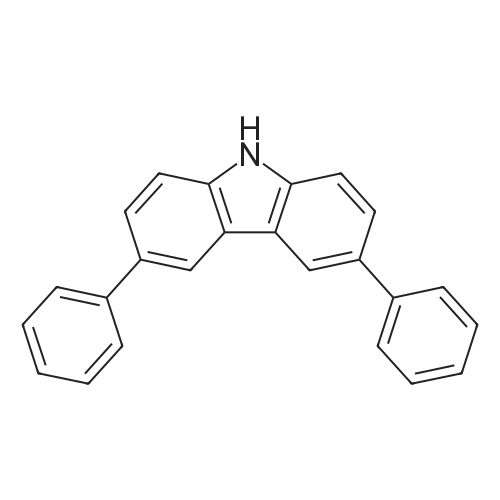
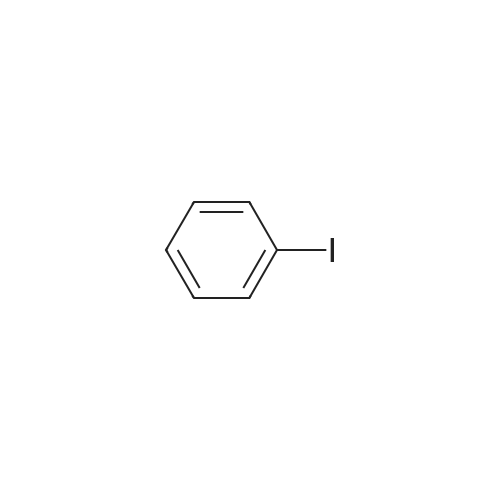
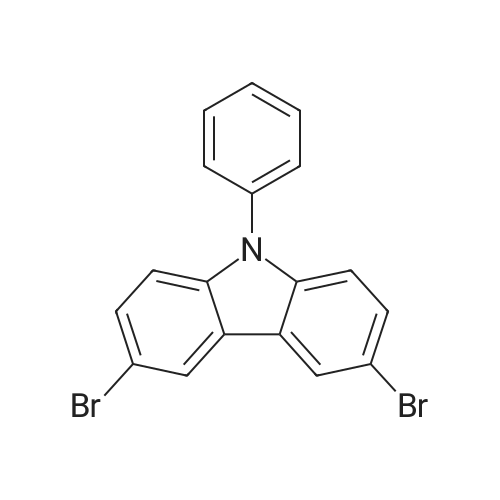
Example 10; a) 3.09 g (9.23 mmol) of 3,6-dibromocarbazole and 2.78 g (22.2 mmol) of phenylboronic acid are stirred in 140 ml of THF under argon. 12.76 g (92.30 mmol) of potassium carbonate in 46 ml of water are added. 0.27 g (0.23 mmol) of palladium tetrakis(triphenylphosphine) are added under argon and the reaction is stirred under reflux over night. At room temperature, a 1 % aqueous solution of NaCN is added to the medium which is boiled for 20 min. At room temperature, ethyl acetate is added and the organic phase is washed with water, dried over magnesium sulfate, filtered and evaporated under reduced pressure. The crude material is purified by chromatography over silica gel (cyclohexane/ethyl acetate 4:1 ) to give 2.17 g of a white solid (74%). 1H NMR (CDCI3, 300 MHz) 8.34 (d, J = 1.8 Hz, 2H), 8.11 (s, 1 H), 7.74- 7.68 (m, 6H), 7.52-7.45 (m, 6H), 7.37-7.32 (m, 2H). |
| 74% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In toluene; at 80℃; for 8h;Inert atmosphere; |
In the nitrogen atmosphere, 3,6-dibromocarbazole (5 g, 15.4 mmol) was added sequentially to the flask, Phenylboronic acid (4.1 g, 33.9 mmol) (Triphenylphosphine) palladium (0.7 g, 0.6 mmol) Toluene (45 ml), 2M sodium carbonate (45 ml), And the mixture was stirred at 80 C for 8 hours. After separating the organic phase, Concentrated under reduced pressure in an evaporator. The resulting residue was purified by silica gel column chromatography, 3,6-diphenylcarbazole (3.6 g, yield 74%) was obtained. |
| 74% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In toluene; at 80℃; for 8h;Inert atmosphere; |
In the nitrogen atmosphere,3,6-dibromocarbazole (5 g, 15.4 mmol) was added sequentially to the flask,Phenylboronic acid (4.1 g, 33.9 mmol)(Triphenylphosphine) palladium (0.7 g, 0.6 mmol)Toluene (45 ml),2M sodium carbonate (45 ml),And stirred at 80 C for 8 hours.After separating the organic phase,Concentrated under reduced pressure in an evaporator.The resulting residue was purified by silica gel column chromatography,3,6-diphenylcarbazole (3.6 g, yield 74%) was obtained. |
| 73% |
With potassium carbonate;tetrakis(triphenylphosphine) palladium(0); In toluene; for 10h;Heating / reflux; |
Preparation of 3,6-diphenylcarbazole Reaction 5As depicted in Reaction 5, 15g (46 mmol) of 3,6-dibromocarbazole, 14g (92 mmol) of phenylboronic acid, K2CO3 (2 mol), Pd(PPh3)4 and toluene were refluxed for 10 hours. The reaction mixture was extracted with methylene chloride. The solvent was removed, and then the residue was purified by column chromatography (eluent: hexane) to give the title compound. Yield: 73%. MS (El) (Calcd. for C24H17N: 319.14, Found: 319). |
| 69% |
With palladium diacetate; potassium carbonate; triphenylphosphine; In tetrahydrofuran; water;Reflux; |
First, 100 g (310 mmol) of 3,6-dibromocarbazole (CAS 6825-20-3) E1 together with 189 g (1550 mmol) of phenylboronic acid (CAS 98-80-6) E2And 430 g (3.1 mol) of potassium carbonate was introduced into a 41 four-necked flask and dissolved in 1000 ml of tetrahydrofuran and 300 ml of water.After the mixture had been degassed for 30 minutes, 140 mg (0.62 mmol) of palladium acetate and 650 mg of triphenylphosphine were added, and the mixture was heated overnight under reflux.700 ml of water was then added to the batch and the aqueous phase was extracted several times with dichloromethane.The combined organic phases were dried over sodium sulfate and the solvent mixture was removed in vacuo.The resulting residue was dissolved in 1.5 mL of dichloromethane and filtered.The solvent was again removed in vacuo and the solid was washed with stirring with 600 mL of ethanol.Filtration and drying gave 68 g (0.21 mol, 69%) of desired material. |
| 68% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80℃; |
Carbazole (16.72g, 100mmol), NBS (59.4g, 210mmol), BPO (2.42g, 10mmol), methylene chloride (300 ml) at room temperature is injected into the medal and then 2000. When reaction is completed methylene chloride and water, NaHCO 3 extracted using aqueous solution obtained after the organic layer Na 2 SO 4 dried to, for the products and re-crystallized concentrating 23.73g (73%)obtained. Said product obtained (23.73g, 73.02mmol), phenylboronic acid (19.6g, 160.64mmol), Pd (PPh 3) 4, (8.44g, 7.3mmol), K 2 CO 3 (60.55g, 438.12mmol), THF (320 ml), water (160 ml) in 80 C agitation return current doesn't have any error frames, turns on the light. When reaction is completed for reducing temperature a high temperature to the normal temperature after methylene chloride and a water extraction of the organic layer obtained after MgSO 4 dried to, for the products and re-crystallized concentrating 15.9g (68%)obtained |
| 63% |
With potassium carbonate;palladium diacetate; tris-(o-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 80℃; for 3.5h; |
As an example of a material according to the invention, a synthetic method of a compound represented by formula (59) 9-[4-(3, 6-diphenyl-N-carbazolyl)] phenyl- 10-phenylanthracene (hereinafter referred to as DPCzPA) below will be described. [0209][0210] This compound is prepared in accordance with the synthetic method shown below. Note that 9-phenyl-10-(4-bromophenyl) anthracene is prepared in the manner shown in Example 1. [0211]First, a synthetic method of 3,6-dibromocarbazole will be shown below. A mixture of 6.5 g (20.0 mmol) 3,6-dibromocarbazole, 5.0 g (41.0 mmol) of phenylboronic acid, 93 mg (0.40 mmol) of palladium acetate, 6.9 g (5.2 mmol) of potassium carbonate, water (25 mL), 610 mg of tri(ortho-tolyl) phosphine, and dimethoxyethane (50 mL) is heated to reflux at 80 0C for 3.5 three hours. After the reaction, the solution is rinsed with water, aqueous layer is extracted with toluene, and it is rinsed together with the organic layer using saturated salt solution, and thereafter EPO <DP n="67"/>dried with magnesium sulfate. After natural filtration, the filtrate is condensed to obtain 4.1 g of 3,6-di(2-phenyl-phenyl)-carbazoleas a white solid at a yield of 63 %. A synthetic scheme of 3,6-di(2-phenyl-phenyl)-carbazoleis shown below. |
| 63% |
With potassium carbonate;palladium diacetate; Tri(p-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 80℃; for 3.5h; |
[Step 2] A synthesis method of 3,6-diphenylcarbazole is described.; A synthesis scheme of 3,6-diphenylcarbazole is represented by (a-2). [Show Image] 6.5 g of 3,6-dibromocarbazole (20 mmol), 5.0 g of phenylboron acid (41 mmol), 93mg of palladium(II) acetate (0.40 mmol), 610mg of tri(ortho-tolyl)phosphine (1.9mmol) were put into a 200mL three-necked flask, and then, the inside of the flask was substituted by nitrogen. Into the mixture, 50 ml of ethyleneglycol dimethylether (abbreviation : DME), and 25 mL of a pottassium carbonate water solution (2.0 mol/L) were added. This mixture was refluxed for 3.5 hours at 80 C. After the reaction, the reaction mixture was washed with water and a water layer was extracted with toluene. The extracted solution and an organic layer were washed with a saturated saline, and dried by magnesium sulphate. The mixture was naturally filtrated. The filtrate was condensed, and 4.1 g of an objective matter, a white powder solid was obtained at the yield 63 %. |
| 63% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 17h;Inert atmosphere; |
A mixture of 3,6-dibromo-9H- carbazole (3.0 g, 9.2 mmol) (Aldrich), phenylboronic acid (3.0 g, 25 mmol) (Aldrich), tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) (0.3 g, 0.26 mmol) (Frontier Scientific, Logan, UT) and potassium carbonate (6.5 g, 47 mmol) (Aldrich) in dioxane/water (75 mL/15 mL) (Aldrich) was degassed with bubbling argon (Airgas, San Marcos, CA) for 60 min and heated at about 100 C on a hot plate with a silicone oil bath for about 16 hours. Upon cooling to room temperature, the whole mixture was mixed with ethyl acetate (Aldrich) and rinsed with brine. Then, the organic mixture was dried over Na2S04 (Aldrich), loaded on silica gel (Grade 135) and purified by flash column using eluents of ethyl acetate/hexanes (10% to 30%) (Aldrich). After removal of solvents, a white solid (Compound OSC-3) was obtained (2.1 g, in 63% yield). Confirmed by 1 HNMR (Jeol Instruments, Peabody, MA). |
| 63% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 16h; |
A mixture of 3,6-dibromo-9H-carbazole (3.0 g, 9.2 mmol), phenylboronic acid (3.0 g, 25 mmol), Pd(PPh3)4 (0.3 g, 0.26 mmol) and potassium carbonate (6.5 g, 47 mmol) in dioxane/water (75 mL/15 ml) was degassed and heated at about 100 C for about 16 hours. Upon cooling to room temperature, the whole was worked up with ethylacetate/brine, the organic was dried over Na2So4, loaded on silica gel and purified by flash column using eluents of ethyl acetate/hexane (10% to 30%). After removal of solvents, a white solid (Compound 11 ) was obtained (2.1 g, in 63% yield). |
| 48% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80℃; for 10h;Inert atmosphere; |
30 g (92.3 mmol) of 3,6-dibromo-9H-carbazole was dissolved in 0.3 L of tetrahydrofuran (THF) in a nitrogenenvironment, 28 g (231 mmol) of phenyl boronic acid and 3.2 g (2.8 mmol) of tetrakis(triphenylphosphine)palladium wereadded thereto, and the mixture was agitated. 40.8 g (277 mmol) of potassium carbonate saturated in water was addedthereto, and the mixture was heated and refluxed at 80 C for 10 hours. When the reaction was terminated, water wasadded to the reaction solution, and the mixture was extracted with dichloromethane (DCM) and treated with anhydrousMgSO4 to remove moisture and then, filtered and concentrated under a reduced pressure. The obtained residue wasseparated and purified through flash column chromatography, obtaining a compound I-4 (14.3 g, 48 %). HRMS (70 eV, EI+): m/z calcd for C24H17N: 319.1361, found: 319.1. HRMS (70 eV, EI+): m/z calcd for C24H17N: 319.1361, found: 319.1. |
| 33% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In water; N,N-dimethyl-formamide; at 80℃; for 4h;Inert atmosphere; |
General procedure: Under nitrogen protection system, 3-bromo -9H- carbazole 1.6mmol, tetrakistriphenylphosphine palladium 0.64mmol, anhydrous carbonatePotassium1.92mmol phenylboronic acid 1.92mmol, was dissolved in N, N- dimethylformamide: H2O = (15ml: 2ml) mixed solvent,80 stirring for 4h, after completion of the reaction, cooled to room temperature, the mixed solution was extracted with dichloromethane and water, the organic phase was dried over anhydrous magnesiumDried and evaporated, evaporated and the product after column chromatography to obtain 3-phenyl -9H- carbazole (A-1) 1.44mmol, 90% yield. The method of the same A-1, by column chromatography to obtain a white solid product 3,6-diphenyl -9H- carbazole (A-2), producingRate of 33%. |
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In 1,2-dimethoxyethane; ethanol; water; for 18h;Heating / reflux; |
EXAMPLE I Synthesis of 3,6-diphenyl carbazole In a 250 milliliter round bottom flask there were added 3,6-dibromocarbazole (5 grams), 50 milliliters of 1,2-dimethoxyethane, phenylboric acid (4.8 grams) dissolved in ethanol, and sodium carbonate (4.2 grams) dissolved in 20 milliliters of water.. After the resulting solution was saturated with argon, 0.49 gram of tetrakis-(triphenylphosphine) palladium was added.. The reaction mixture was heated to reflux and stirred for 18 hours.. The reaction flask was removed from the heat and cooled to room temperature, about 22 C. to about 25 C. The resulting solution was transferred to a separatory funnel, and the organic layer, which contained product, was separated from the aqueous phase.. After removal of the organic solvents by evaporation, the residue was subjected to column chromatography on silica gel to yield 3.5 grams of 3,6-diphenyl carbazole as colorless powder product.. Its chemical structure was confirmed by Proton IR analysis. |
|
|
According to an ordinary process, 3,6-diphenylcarbazole was synthesized by reacting 3,6-dibromocarbazole and phenylboronic acid. |
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In ethanol; toluene; |
Manufacturing Example 8 A solution was prepared by adding 12.7 g of 3,6-dibromocarbazole, 10.0 g of phenylboronic acid, and 2.61 g of tetrakis(triphenylphosphine)palladium into 40 ml of ethanol and 170 ml of toluene. The resulting solution was blended with 90 ml of a 22% aqueous solution of sodium carbonate, and the mixture was heated under reflux in a nitrogen atmosphere for 5 hours. Insoluble materials were removed therefrom by performing hot filtration using a filter aid. Thereafter, the organic layer was separated from the aqueous layer, and the solvent was distilled off under a reduced pressure. After washing with water and drying were performed, a light brown powder was prepared. The resulting powder was subjected to a column chromatography treatment (eluant: toluene), followed by washing with hexane. Thus, 6.0 g of a colorless needle crystal of 3,6-diphenylcarbazole was produced. The physical properties thereof were as follows. melting point: 180.5 C. to 181.5 C. elemental analysis value (%): measured value/calculated value C 90.44/90.24 H 5.25/5.38 N 4.31/4.39 infrared absorption spectrum (KBr pellet method) NH stretching vibration: 3423, 3378 cm-1 |
| 38 g |
|
3,6-dibromocarbazole (50 g, 0.154 mol), phenylboronic acid (41 g, 0.336 mol), 150ml water, 80g sodium carbonate, and 600ml dioxane were charged into a 2 liter pot with magnetic stirrer, reflux condenser and nitrogen inlet, and sparged with nitrogen for one hour. Pd2DBA3 (6g, 0.0066mol) and tri-t-butylphosphine (3 g, 0.0148mol) was quickly added from the drybox. The reaction was refluxed overnight. The next day water was added to the reaction mixture and methylene chloride extractions were preabsorbed to 141 g of activated silica and purified by column chromatography using methylene chloride/hexanes yielding 38 grams of product 3,6-diphenylcarbazole. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping