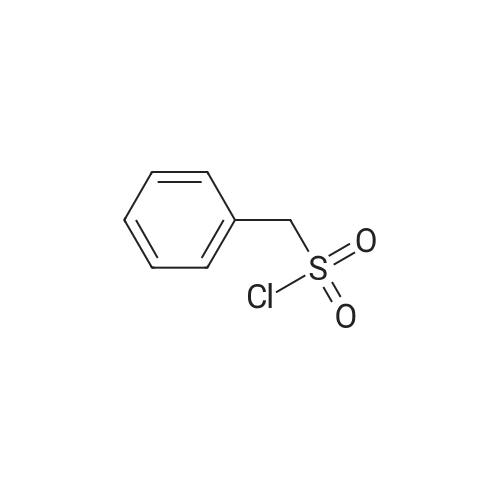
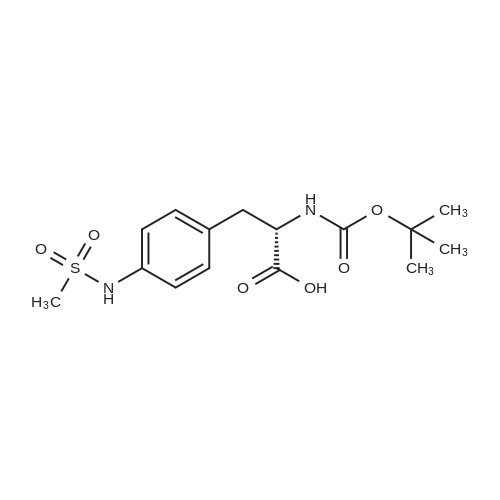
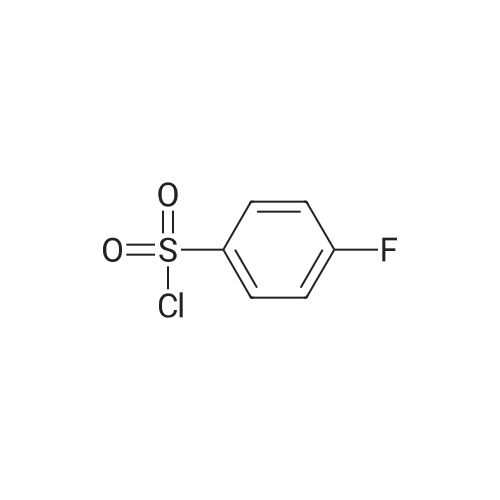
|
|
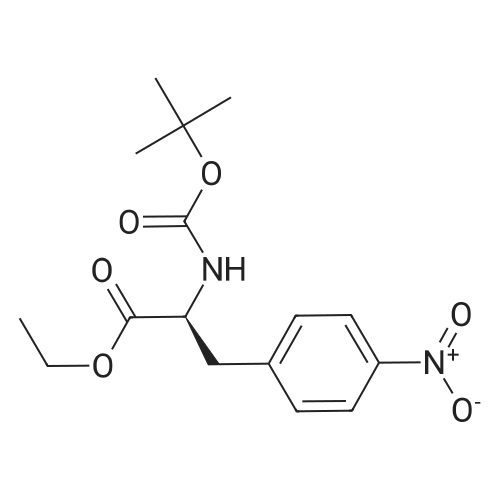
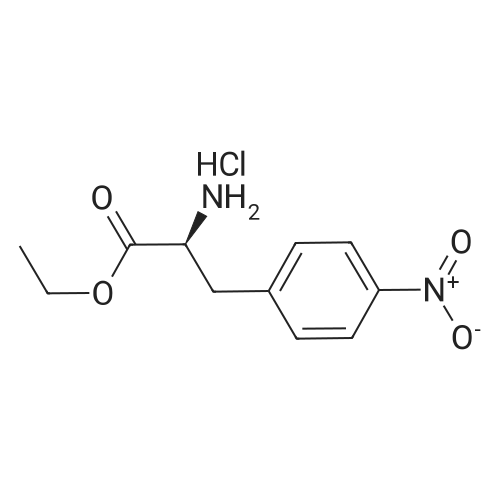
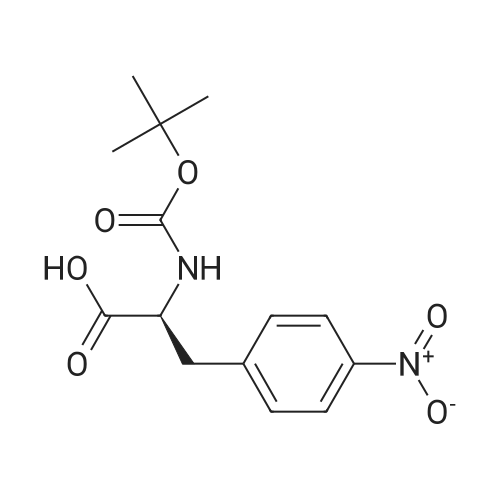
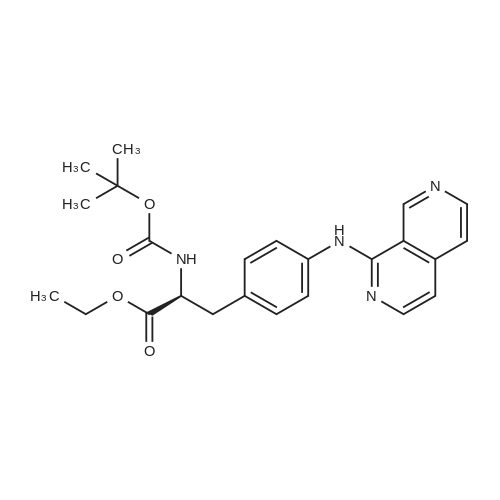
A solution of (S)-ethyl-3-(4-aminophenyl)-2-(t-butoxycarbonylamino)propionate (130.8 g, 0.425 mol) in DCM (800 ml) was cooled to 0 and treated with NMM (56.0 ml, 0.51 mol), stirred 5 minutes and then a solution of the acid chloride (98.3 g, 0.468 mol) in DCM (200 ml) was added dropwise keeping the reaction temperature below 5.. The reaction was stirred for 1 h, quenched with NaHCO3 solution (500 ml), the organic layer separated, washed with NaHCO3 solution (500 ml), 10% citric acid solution (500 ml) and NaHCO3 solution (500 ml), dried (MgSO4) and concentrated in vacuo to give a yellow solid which was recrystallized (EtOAc/Hexane) to give the title compound (140 g, 69%): deltaH (DMSO d6) 8.80 (2H, s), 7.55 (2H, d, J 8.5 Hz), 7.23 (2H, d, J 8.5 Hz), 4.00 (3H, m), 3.40 (2H, br. s), 2.90 (1H, m), 2.80 (1H, m), 1.30 (9H, s), 1.25 (3H, t); m/z (EI+, 70V) 504. |
|
|
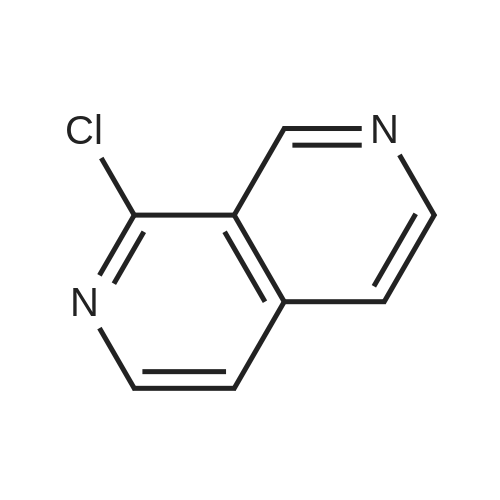
A slurry of the compound of Intermediate 1 (51.2g, 0. 267mol) in DCM [(195MUT)] and thionyl chloride [(195MOI,] 2. [67MOL)] was treated with DMF (5 drops) and heated to reflux for 4h. The reaction was concentrated in vacuo and azeotroped with toluene [(2X50M1)] to give a yellow solid which was used without further purification. A solution of [ETHYL- (S)-3- (4-] aminophenyl)-2- (t-butoxycarbonylamino) propanoate (130.8g, 0. [425MOL)] in [DCM (800ML) WAS COOLED TO 0XB0; AND TREATED WITH NMM 0.51MOL),] stirred for 5 minutes and then a solution of the acid chloride (98.3g, 0. [468MOL)] in DCM [(200ML)] was added dropwise keeping the reaction temperature [BELOW 5XB0;.] The reaction was stirred for 1h, quenched with NaHCO3 solution [(500ML),] the organic layer separated, washed with NaHCO3 [SOLUTION (500ML),] 10% citric acid solution [(500MOI)] and [NAHCO3] [SOLUTION (500M1),] dried [(MGS04)] and concentrated in vacuo to give a yellow solid which was [RECRYSTALLISED] (EtOAc/hexane) to give the title compound, (140g, [69%).] [8H] (DMSO d6), 8.8 (2H, s), 7.55 (2H, d, J 8.5Hz), 7.23 (2H, d, J 8.5Hz), 4.0 (3H, m), 3.4 (2H, b s), 2.9 (1H, m), 2.8 [(1 H,] m), 1.3 (9H, s), 1.25 (3H, t); m/z (ES+, 70V) 504 (MNa+). |
|
With 4-methyl-morpholine; In dichloromethane; at 0℃; for 1.08333h; |
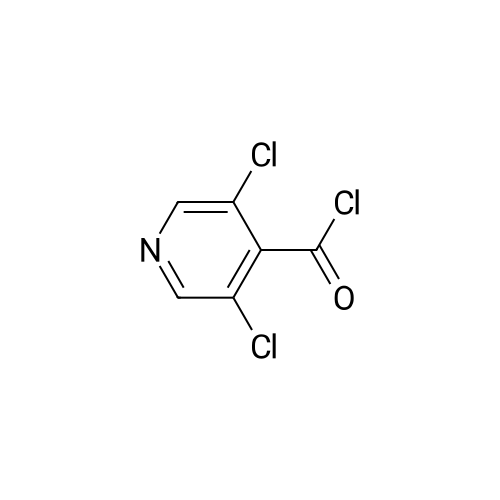
To a nitrogen flushed 500 mL round bottom flask was charged with 3,5-dichloro- isonicotinic acid (46.5 g, 0.24 mol), CH2CI2 (150 mL), DMF (0.5 mL), and thionyl chloride (20 mL,33.9 g 0.28 mol). After the slurry was refluxed for 5 h, additional thionyl chloride (5 mL, 0.70 mol) and CH2CI2 (100 mL) were added, and the reaction was refluxed for additional 45 min. The reaction mixture was concentrated, and the residue was azeotroped with toluene to give the crude acyl chloride, which was used immediately. Thus, the crude acyl chloride was dissolved in CH2CI2 (150 mL) and was added to N-BOC-4-amino-L-phenylalanine ethyl ester (60 g, 0.20 mol) and 4-methylmorpholine (44 mL, EPO <DP n="20"/>0.40 mol) in CH2CI2 (400 mL) at O0C over 5 min. After stirring at 00C for 1 h, the reaction was quenched with dilute aqueous NaHCtheta3. The organic layer was separated and the aqueous layer was extracted with CH2CI2 (500 mL). The organic layers were combined, dried over anhydrous MgSO4 and concentrated in vacuo, and the residue was purified by flash column chromatography on silica gel eluting with 4:1 to 3:2 EtOAc/hexanes to afford the title compound (95 g, 100% yield). lH NMR (400 MHz, CD3OD) delta 8.60 (s, 2H), 7.54 (d, 2H), 7.20 (d, 2H), 4.20-4.36 (m, IH), 4.10 (q, 2H), 3.02-3.12 (m, IH)5 ),2.82-2.92 (in, IH), 1.34/1.30 (s, 9H).1.20 (t, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping