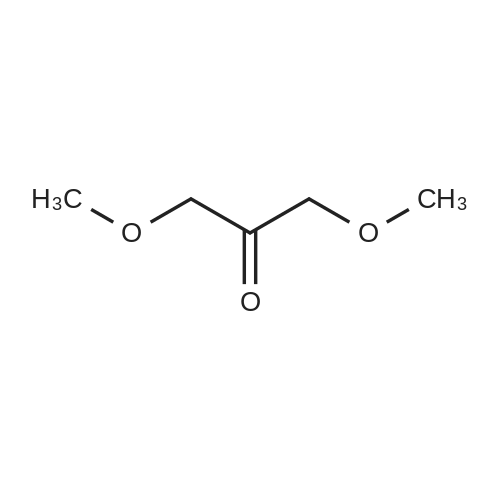
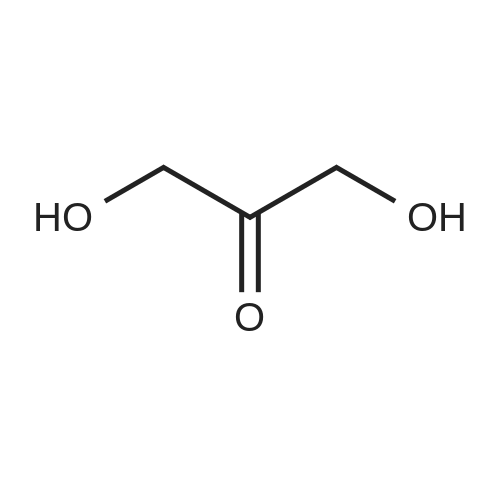
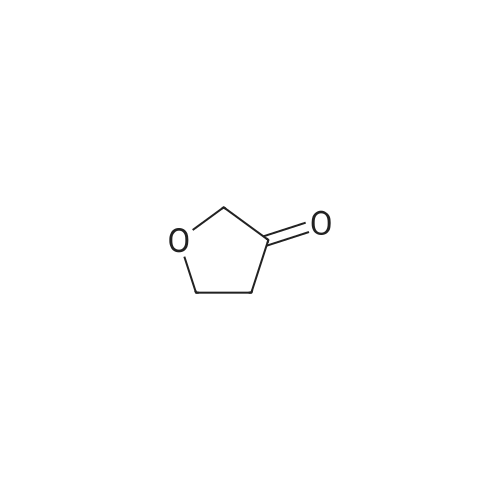
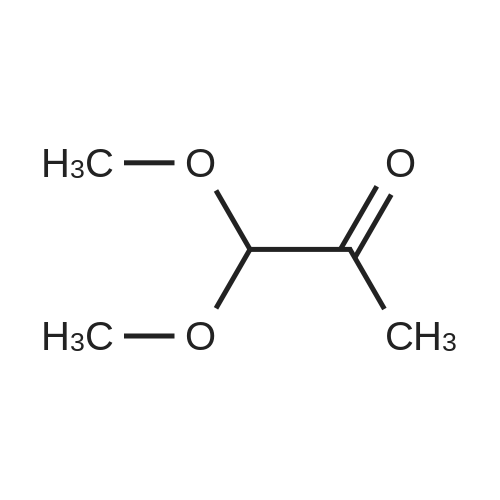
| 82% |
|
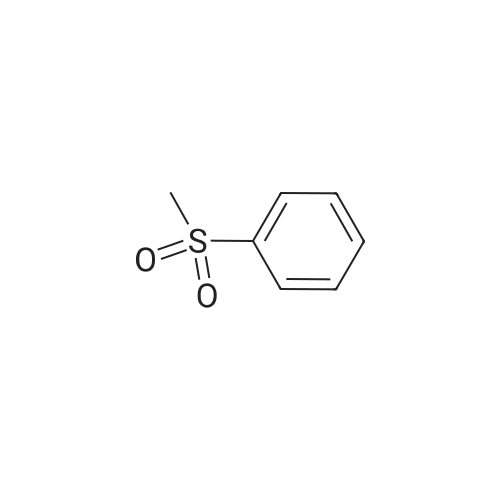
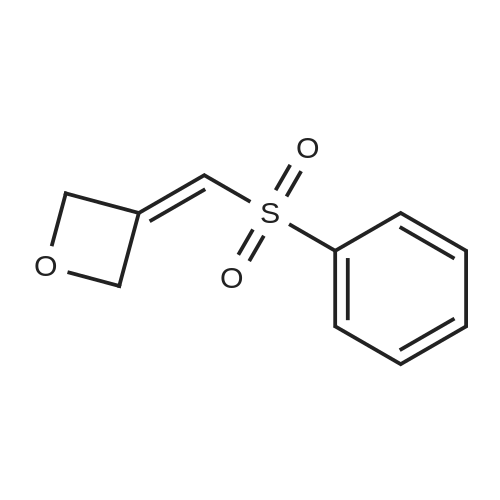
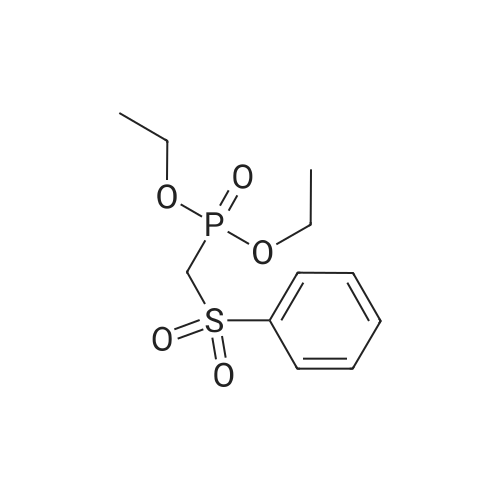
To a solution of (methylsulfonyl)benzene (2.2 g, 13.9 mmol) in THF (38 mL) at 0 was added n-BuLi (2.5 M in hexanes, 12.2 mL, 30.6 mmol) dropwise over 10 minutes. After the mixture was stirred for 30 min, chlorodiethylphosphonate (2.4 mL, 16.7 mmol) was added dropwise to the reaction. After 30 minutes, a solution of oxetan-3-one (1.0 g, 13.9 mmol) in THF (2 mL) was added dropwise to the reaction mixture at -78 . The reaction mixture was stirred at -78 for 2 hours, then diluted with aqueous NH4Cl (100 mL) and extracted with EtOAc (100 mL x 2) . The combined organic layers were concentrated and the residue was purified by silica gel chromatxography column (petroleum ether/EtOAc = 3/1) to give the title compound (2.4 g, 82%) as a colorless oil. 1H NMR (400 MHz, CDCl3) : delta7.90-7.88 (m, 2H) , 7.68-7.64 (m, 1H) , 7.57 (t, J= 7.6 Hz, 2H) , 6.13-6.11 (m, 1H) , 5.66-5.64 (m, 2H) , 5.30-5.27 (m, 2H). |
| 75% |
|
3-((Phenylsulfonyl)methylene)oxetane To an oven-dried vial was added (methylsulfonyl)benzene (0.570 g, 3.65 mmol) and the vial was evacuated with argon 3 times. The dry THF (17 mL) was added and the reaction was cooled to 0 C. The 2.5 M BuLi in hexanes (3.21 mL, 8.03 mmol) was added dropwise and the reaction began to stir at 0 C. and stirred for 45 minutes. The diethyl chlorophosphate (0.528 mL, 3.65 mmol) was then added at 0 C. and the reaction stirred for 30 minutes. The reaction was then cooled to -78 C. and the oxetan-3-one (0.330 mL, 5.15 mmol) was then added dropwise and the reaction stirred for 2 h. The reaction was then warmed to rt and filtered through a silica plug. The reaction was then concentrated onto silica and purified by MPLC (20 min, 0-40% EtOAc:hex) to provide pure 3-((phenylsulfonyl)methylene)oxetane (0.579 g, 2.75 mmol, 75% yield). 1H NMR (400 MHz, CDCl3): delta 7.91-7.87 (m, 2H), 7.69-7.64 (m, 1H), 7.60-7.55 (m, 2H), 6.12 (quintet, J=2.3 Hz, 1H), 5.66-5.63 (m, 2H), 5.30-5.27 (m, 2H). |
| 74% |
|
To a stirred solution of methyiphenylsulfone (3 g, 19.2 mmol) in dry tetrahydrofuran (15 mL) was added n-butyllithium (2.5 M in tetrahydrofuran; 15.4 mL, 38.4 mmol) at 0 C. Thereaction mixture was stirred for 30 mi Diethyl chiorophosphate (4 mL, 27.8 mmol) wasadded, and the mixture was stirred at 0 C for an additional 30 mm. It was then cooled to -78C, and a solution of 3-oxetanone (1.38 g, 19.2 mmol) in dry tetrahydrofuran (3 mL) wasadded. The mixture was stirred at -78 C for 1.5 h and filtered through a silica plug to give 3-((phenylsulfonyl)methylene)oxetane as a white solid (3 g, 74%). ?H NMR (400 MHz, CDC13)oe 7.90 - 7.87 (m, 2H), 7.66 - 7.64 (m, 1H), 7.59 - 7.56 (m, 2H), 6.12 (s, 1H), 5.65 (d, J =6.0Hz, 2H), 5.29 (d, J =5.6 Hz, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +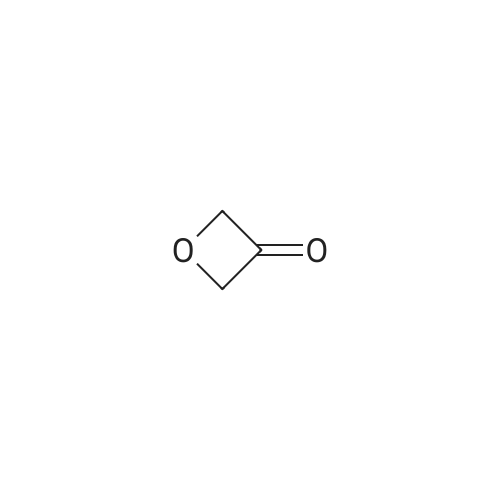

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping