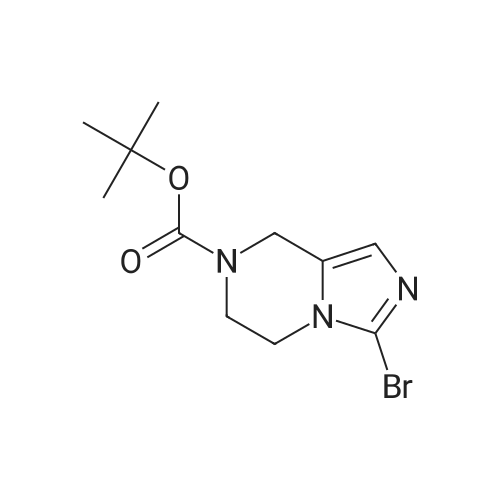
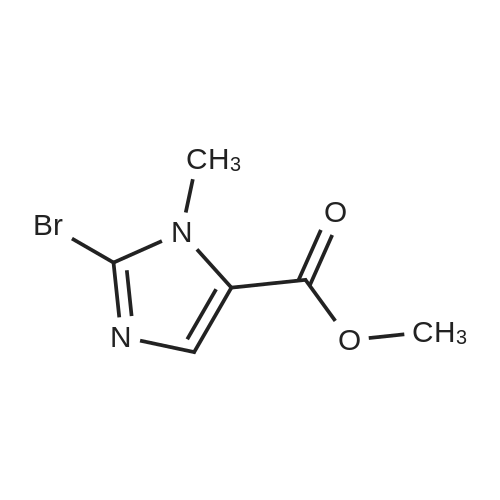
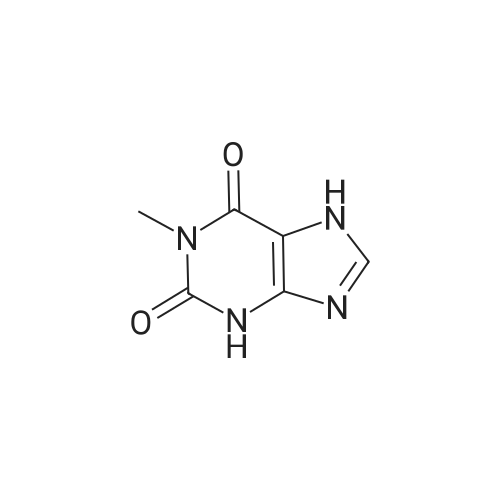
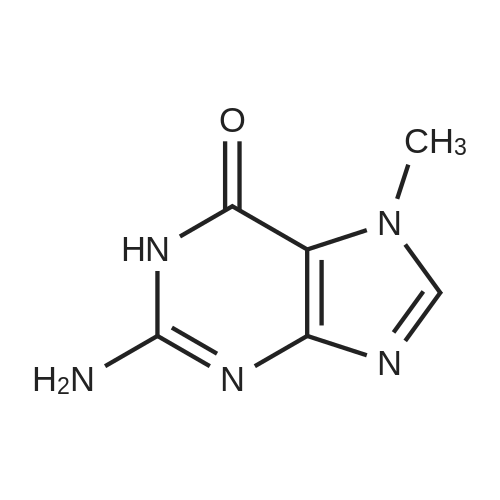
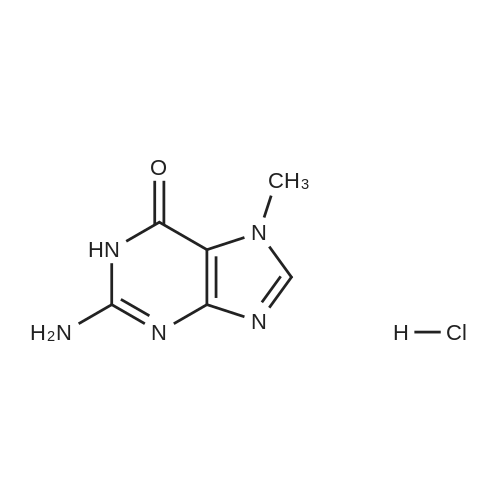
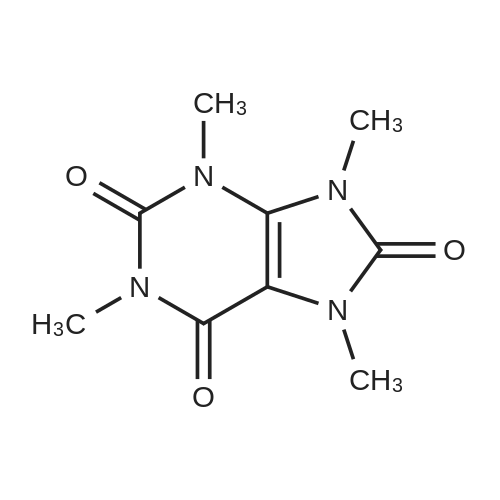
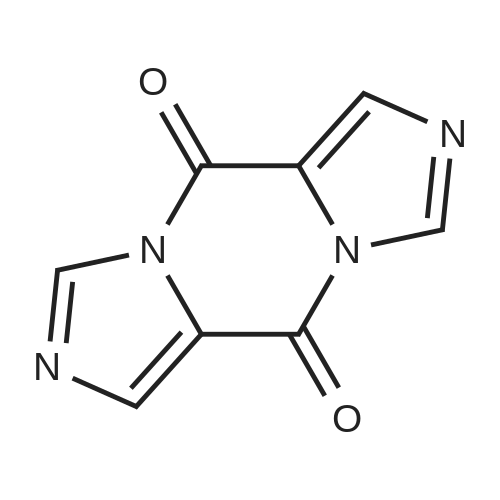
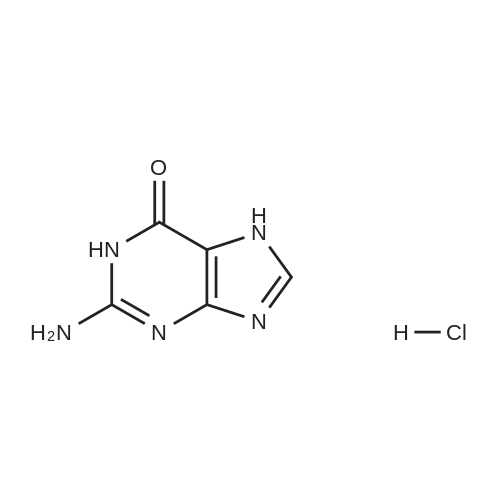
| 86.4% |
With potassium carbonate; In N,N-dimethyl acetamide; at 75 - 95℃;Autoclave; |
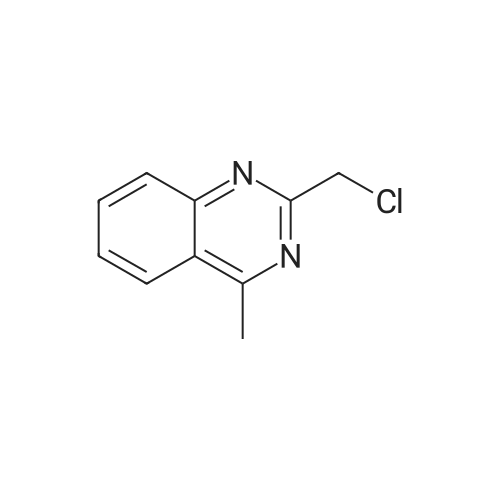
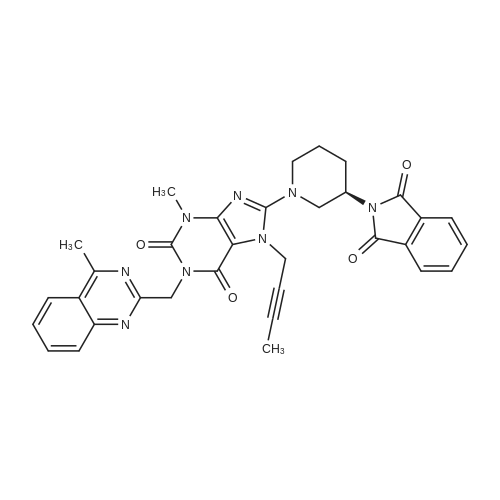
The intermediate (c) is reacted with 2-chloromethyl-4-methylquinazoline (d) to give intermediate (e).Steps:Was charged into a 10 L autoclave550 g (1.851 mol) of intermediate (c),463.3 g (2.405 mol)2-chloromethyl-4-methylquinazoline (d),332.6 g (2.407 mol) of potassium carbonate and 6 L of potassium carbonate(Dimethylacetamide,DMAC).Stirring, heating to 75 ~ 95 C reaction,7 ~ 10h after the end of the reaction,Cooling down to 65 ,Add 3L methanol stirring 0.5 ~ 1h,filter,The filter cake was washed with 1 L of methanol.The resulting filter cake was beaten with 2 L of water,filter,The filter cake was washed with 1 L of water,1 L methanol wash,A yellow filter cake,The product after drying was 724.9 g,The yield was 86.4%Purity 98.5%. |
| 86.4% |
With potassium carbonate; In N,N-dimethyl acetamide; at 75 - 95℃; |
The intermediate (c) is reacted with 2-chloromethyl-4-methylquinazoline (d) to give intermediate (e). Steps: Was charged into a 10 L reaction vessel 550g (1.851mol) of intermediate (c), 463.3 g (2.405 mol) of 2-chloromethyl-4-methylquinazoline (d), 332.6 g (2.407 mol) of potassium carbonate and 6 L of potassium carbonate (in dimethylacetamide, DMAC). Stirring, heating to 75 ~ 95 C reaction, 7 ~ 10h after the end of the reaction, cooling down to 65 deg. C, add 3L methanol stirring 0.5 ~ 1h, filtered, the filter cake was washed with 1 L of methanol. The obtained filter cake was stirred with 2L of water and filtered. The filter cake was washed with 1L of water and 1L of methanol to obtain a yellow filter cake, and dried to obtain 724.9g of product with yield of 86.4% and purity of 98.5%. |
| 85% |
With sodium carbonate; In N,N-dimethyl-formamide; at 80℃; for 12h; |
To a suspension of 3b (849 mg, 3 mmol) and Na2CO3 (382 mg, 3.6 mmol) in DMF (36 mL) was added 2-chloromethyl-4-methylquinazoline (636 mg, 3.3 mmol). The reaction mixture was stirred at 80 C for 12 h. After cooling to r.t., the reaction mixture was diluted with DCM, washed with water and brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The resulting residue was purified by flash chromatography (petroleum ether/ethyl acetate, 1:1) to give 4b as a white solid (1.05 g, 80%). |
| Ca. 84% |
With tetrabutylammomium bromide; potassium carbonate; In dimethyl sulfoxide; at 75 - 80℃; |
700 ml of DMSO, 77.8 g of 2-(Chloromethyl)-4-methyl-quinazoline( 0.4038 moles) , 100 g of 3-methyi-7-(2-butyn-l-yl)-8-bromo-xanthine (0.3365 moles, prepared in Example-2), 0.5 g tetrabutyl ammonium bromide and 55.8 g of anhydrous potassium carbonate (0.4038 moles) were added into a 5 lit a round bottom flask equipped with overhead stirrer & thermo pocket at 20-30C and the temperature was raised to 75- 80C. The reaction mixture was maintained at 75-80C for 2-3 hrs. After completion of the reaction, reaction mixture was cooled to 45-50C. To the reaction mixture was slowly added 600 ml of methanol and stirred for 60 min at 45-50C. The solid was filtered and washed with 200 ml of methanol followed by DM water slurry. The wetmaterial was charged into RB flask and charged 700 ml of methanol into RB flask; the temperature was raised to 65C and maintained for 60 min. The reaction mass was cooled to 40-45C and maintained for 60 minutes. Filtered the solid and washed with 200 ml methanol. The wet material was dried at 40-45C for 5-8 hours to get title compound (128 g, yield -84%, purity > 99 %). |
| 75% |
With potassium carbonate; In N,N-dimethyl-formamide; at 80℃; for 8h; |
In 150 mL of DMF, 2.89 g (15 mmol) of compound 3, 4.46 g (15 mmol) of compound 4, 4.15 g (30 mmol) of potassium carbonate were added, and the reaction was performed at 80 C for 8 hours. The reaction progress was monitored by TLC, suction filtration, and the filtrate was cooled to 0 C, It was left for 2 hours, filtered, washed with water, and dried to obtain 8-bromo-7- (2-butyn-1-yl) -3,7-dihydro-3-methyl-1-[(4-methyl-2 -Quinazolinyl) methyl] -1H-purine-2,6-dione 5.12 g (11.3 mmol) with a yield of 75%. |
| 12.1 g |
With potassium carbonate; In N,N-dimethyl acetamide; at 98℃; for 8h; |
3-Methyl-7-(2-butyn-l-yl)-8-bromo-xanthine (10 gm) and N,N-dimethylacetamide (150 mL) were charged into a 1 000 mL round bottomed flask equipped with a mechanical stirrer. Potassium carbonate (10.7 gm) and 2-(chloromethyl)-4- methylquinazoline (7.1 gm) were added to the reaction mixture at room temperature. The reaction mixture was heated to 98 C and maintained the temperature for 8 hours. The reaction mixture was cooled to 30C and water (450 mL) was added and the mixture was stirred for 1 hour at 30C. The solid formed was collected by filtration and washed with water (150 mL). The wet cake was charged into 500 mL round bottomed flask and toluene (220 mL) was added and the mixture was heated to reflux temperature and maintained for 1 hour. The mixture was cooled to 10C and maintained for 3 hours. The solid was collected by filtration and washed with toluene (5 mL). The solid was dried in oven under vacuum at 77C to get 12.1 gm of the title compound. Purity by HPLC: 98.22%. |
|
In 1-methyl-pyrrolidin-2-one; toluene;Alkaline conditions; |
Example 35 Preparation of 8-bromo-7-(but-2-ynyl)-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione 3-Methyl-7-(2-butine-1-yl)-8-bromoxanthine was reacted with 2-(chloromethyl)-4-methylquinazoline in the presence of base under phase transfer catalyst using a N-methyl pyrrolidone/toluene mixture as the reaction solvent. The reaction mixture was heated overnight. When the reaction was complete, the reaction mixture was cooled to ambient temperature. A solid precipitate formed and was separated by filtration and washed with toluene and then with water to provide the product, 8-bromo-7-(but-2-ynyl)-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione having more than 97% purity. |
| 253 g |
With potassium carbonate; In N,N-dimethyl acetamide; at 80 - 85℃; for 10h; |
To the stirring mixture of purine (200g) in dimethylacetamide (1400 ml) were added quinazoline (147g), potassium carbonate powder (140 g) at 25-30 C. The reaction mixture was heated to 80-85C for 10 hr. To the reaction mixture, water was charged (5600 ml). After addition of water, reaction mixture was cooled to 25-30C and maintained for 30 min. Filtered reaction mixture to give bromopurine (282 g) which is further purified from dimethyl formamide to give pure bromopurine (253 g). HPLC Purity: 99.47% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 95 - 100℃; for 3h; |
In a round bottom flask, 140 ml DMF, 20 gm of 8-bromo-7-but-2-yn-1-yl-methyl-3,7-dihydro-1H-purine-2,6-dione and 15.67 gm of 2-(chloromethyl)-4-methyl-1,2-dihydroquinazoline were charged. 14 gm potassium carbonate was added to the reaction mass and heated to 95-100 C. for 3 hours. The reaction mass was cooled to 0-10 C. & added 100 ml water. The reaction mass was allowed to come to 25-30 C. The solid obtained was filtered & the slurry was washed with water. The obtained solid was purified in ethyl acetate to get the product. Dry wt 25 gms, HPLC purity=98% |
| 105.5 g |
In N,N-dimethyl-formamide; at 95 - 105℃; for 3h; |
In a 2 L three-necked flask was added 60.0 g (0.245 mol) compound a (8-bromo-3-methylxanthine), 32.6 g (0.245 mol) of compound b (1-bromo-2-butyne), 63.3 g (0.490 mol) of diisopropylethylamine, and 570 g of N,N-dimethylformamide. Heating to 95-105C, the mixture was stirred for 4 hours. TLC monitoring until no 8-bromo-3-methylxanthine (Rf (8-bromo-3-methylxanthine) = 0.4, Rf (reaction solution) = 0.6, developing solvent: toluene / absolute ethanol = 6/1, volume ratio). 47.2 g (0.245 mol) of compound d (2-(chloromethyl)-4-methylquinazoline) was added. The mixture was further stirred at 95-105C for 3 hours. TLC monitoring until no compound c (8-bromo-7-but-2-yn-1-yl-3-methyl-3,7-dihydro-1H-purine-2,6-dione) (Rf (compound c) = 0.6, Rf (reaction solution) = 0.7, developing solvent: toluene / absolute ethanol = 6/1, volume ratio). The reaction solution was cooled to 5-25C. To the system, 600 g of tap water was slowly added dropwise. Stirred at 15-25C for 0.5 hours. Filtered and rinsed with toluene. The resulting solid was dried in vacuo at 70C to give compound e (8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione): 105.5 g, HPLC purity 99.9%, yield 95%. |
|
With potassium carbonate; In 1-methyl-pyrrolidin-2-one; at 80℃; |
To a 3000 mL glass vessel equipped with a stirrer, condenser and a thermometer probe were added Formula I (100.0 g, 0.33 mol), Formula 11(70.02 g, 0.36 mol), potassium carbonate (51.16 g, 0.37 mol) and N-Methyl-2-pyrrolidone (500.0 mL, 5.00 vol) and the mass was heated to 80±2 C. The reaction mass was maintained at 80±2 C under stirringfor 6 to 8h. The reaction mass was cooled to 25±5 C and water (1000 mL) was added to the reaction mass under constant stirring. The mass was filtered and the solid was washed with water (200 mL) followed by Methanol (200 mL), suck dried and dried at 45±5 C under vacuum for 8- lOh to obtain compound of Formula III as a pale yellow solid. It is further purified using a mixture of methanol and MDC. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping