|
|
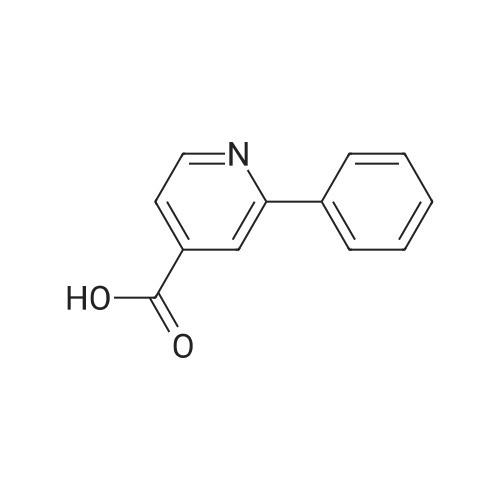
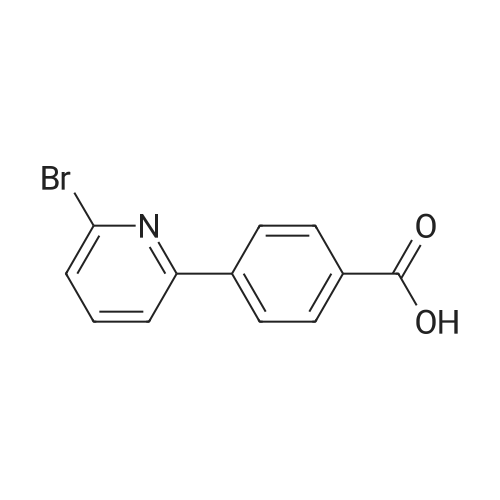
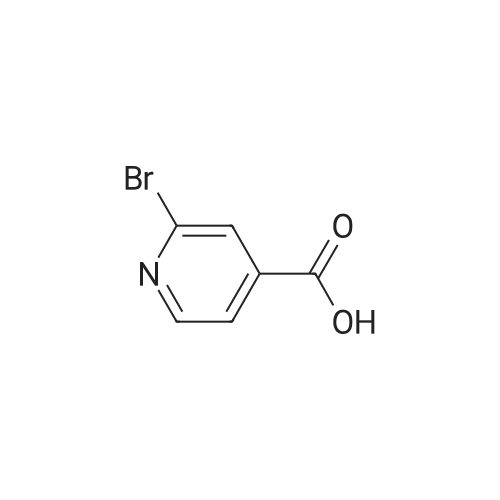
Preparation H1: Synthesis of lithium 2-phenylisonicotinate, N-oxide Preparation H1, Step 1: A mixture of 2-bromo-4-pyridinecarboxylic acid (1.1 g, 5.45 mmol), phenylboronic acid (1.3 g, 10.9 mmol), 2.0 M potassium phosphate (aq) (8.2 mL, 16.34 mmol), and DMF (10 mL) in a 20 mL microwave tube was degassed under vacuum/Ar. A catalytic amount of tetrakis(triphenylphosphine)palladium(0) was added to the tube, the mixture was degassed again, the tube was sealed, and the reaction was heated at 150 C. in the microwave for 30 min. The reaction mixture was filtered, the filtrate was concentrated in-vacuo, and the residue was dissolved in water (10 mL). The mixture was acidified to pH=6 with the addition of 1.0 N HCl, and the resulting precipitate was collected by filtration, rinsed with two portions of ice-cold water, and air dried to yield 575 mg of 2-phenylisonicotinic acid as an off-white solid. MS (ES+)=200 (M+H+). |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,2-dimethoxyethane; water; at 95℃; for 18h;Inert atmosphere; |
Stepl : 2-phenylisonicotinic acid 2-bromoisonicotinic acid (0.210 g, 1 .040 mmol) was dissolved in degassed DME (Volume: 8 ml) under nitrogen. Tetrakis(triphenylphosphine)palladium(0) (0.060 g, 0.052 mmol) was added, the resulting reaction mixture was stirred for 15min.Then aqueous potassium carbonate (4.16 ml, 8.32 mmol) and phenylboronic acid (0.171 g, 1 .403 mmol) were added subsequently. The resulting RM was refluxed at 95 C for 18h and then cooled to rt. After filtration over celite the reaction mixture was acidified to pH 3-4 and the white precipitate was filtered off and washed with water. This resulted in a white powder after recrystallization from 2-methoxyethanol. Yield: 0.1 06 g, 57%. 1 H NMR (400 MHz, DMSO-c): delta 7.44 - 7.60 (m, 3H), 7.71 - 7.86 (dd, J = 4.9, 1 .5 Hz, 1 H), 8.05 - 8.19 (m, 2H), 8.23 - 8.35 (t, J = 1 .2 Hz, 1 H), 8.79 - 8.93 (dd, J = 5.1 , 0.8 Hz, 1 H), 13.56 - 13.97 (s, 1 H). UPLC I (ESI) Rt 1 .37 min, m/z 200.5 [M+H]+ (92%). - - |
|
|
2-Bromoisonicotinic acid (2.02 g, 10 mmol) was dissolved in DMF (80 mL) under argon. Pd(PPh3)4 (0.6 g, 0.52 mmol) was added, and the reaction mixture was stirred at room temperature for 15 min. Na2CO3 (aq. 2N, 40 mL) was then added, followed by the addition of phenylboronic acid (1.67 g, 13.7 mmol). The reaction mixture was heated at 95C (18 h), cooled to room temperature and filtered through <n="76"/>a celite pad. Water (80 mL) was added, and the mixture was acidified with HCl (2 N) to pH = 4. The precipitate was collected via filtration and rinsed with water (2 x 7 mL). The crude product was recrystallized from 2-methoxylethanol to give 2- phenylisonicotinic acid as a grey solid (1.2 g). 1HNMR (DMSO-d6, 400 MHz): delta 7.51 (m, 3H), 7.78 (d, J=4.8 Hz, 1H), 8.13 (t, J=1.6 Hz, 2H), 8.29 (s, 1H), 8.85 (d, j=4.8 Hz, 1H), 13.73 (bs, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping