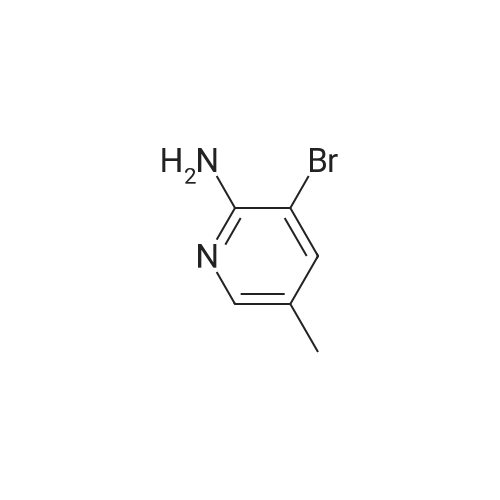
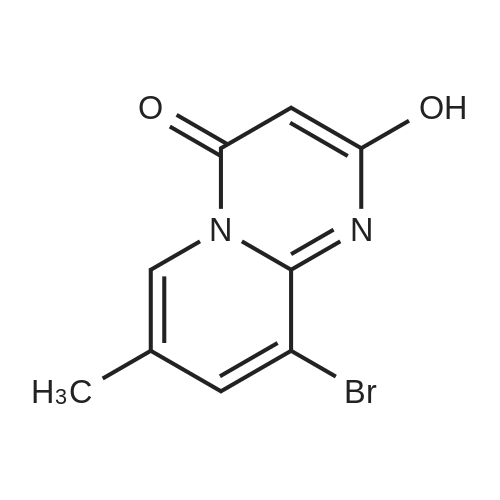
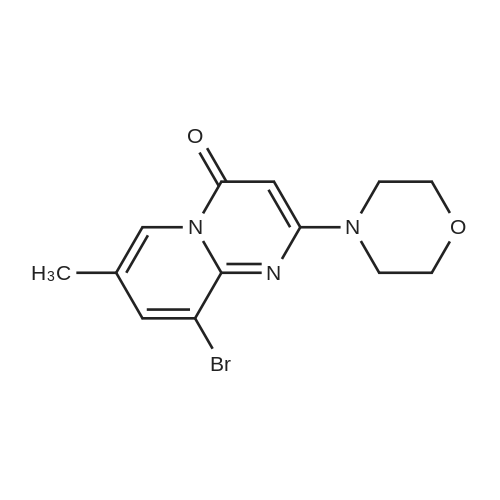
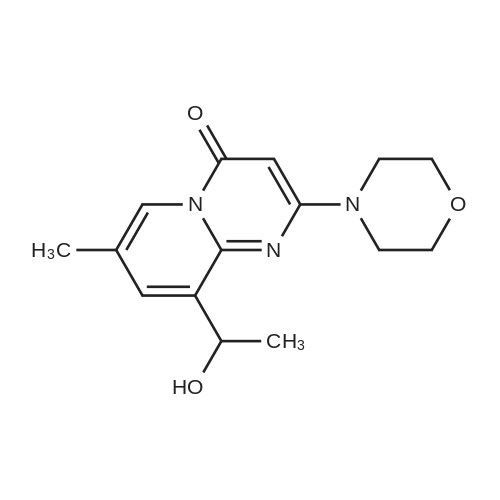
| 60% |
Stage #1: for 4 h; Heating / reflux
Stage #2: With sodium tetrahydroborate In toluene at 0 - 20℃; for 1 h; |
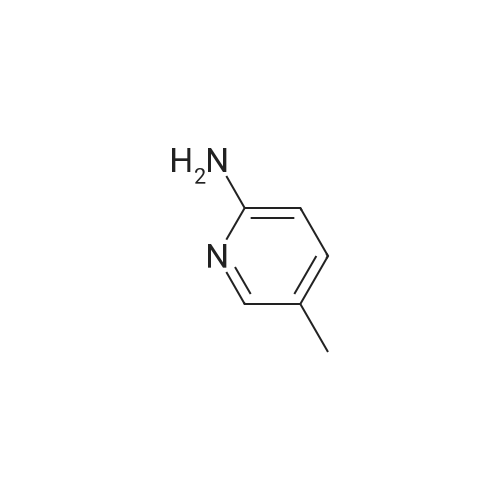
To a suspension of ketone 4 (1 mmol) in toluene (10 mL) was added aniline (3 mmol) and refluxed for 4 h. The reaction mixture was gradually cooled and sodium borohydride (1 mmol) was added at ice-cold temperature. Then reaction mixture was further stirred for 1 h at room temperature. The solution was diluted with dichloromethane (30 mL), the organic layer was washed with water, brine and dried over [NA2S04.] After concentration in vacuo, the residue was purified by column chromatography (silica gel, 3: 1 ethylacetate, petroleum ether) to give pale yellow solid (>60percent yield). 1H NMR (300 MHz, [CDC13)] [6] 8.65 (s, 1H), 7. 58 (s, 1H), (7.11 br t, 2H), 6.68 [(T,] [J=7.] 5 Hz, 1H), 6.46 (br t, 2H), 5.66 (s, 1H), 5.12 (m, 1H), 4.24 (br s, -NH, 1H), 3. 80 (m, 4H), 3.68 (m, 4H), 2.26 (s, 3H), 1.57 (d, J= 6. 7 Hz, 3H). |
| 34% |
Stage #1: With toluene-4-sulfonic acid In toluene for 4 h; Dean-Stark; Reflux
Stage #2: With sodium tetrahydroborate In methanol; toluene at 0℃; |
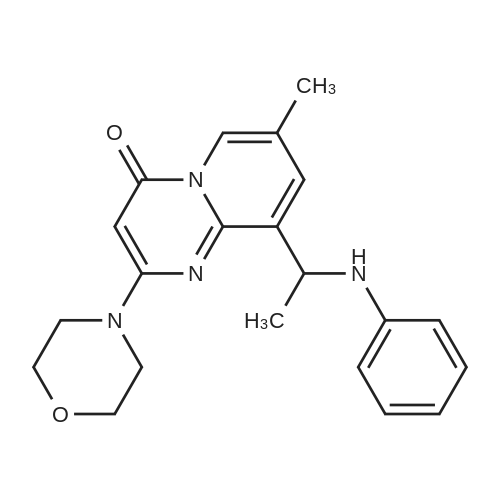
Using a modified procedure outlined by Jackson et al.5,6 9-acetyl-7-methyl-2-(4-morpholinyl)-4H-pyrido[1,2-a]pyrimidin-4-one (8) (4.87 g, 17 mmol), aniline (4.80 mL) and p-toluenesulfonic acid mono hydrate (3.23 g, 17 mmol were suspended in toluene (150 mL) and submitted to Dean-Stark distillation for 4 h. The solution was reduced to 20 mL and diluted with methanol (130 mL). The solution was cooled to 0 °C and sodium borohydride (660 mg, 51 mmol) was cautiously added and left stirring overnight. The solution was concentrated in vacuo and diluted with ethyl acetate and acidified with acetic acid to destroy unreacted sodium borohydride. The solution was neutralised with ammonia, and the phases separated and the aqueous layer was extracted with ethyl acetate. The combined organic extracts were washed with water, brine, and dried with sodium sulfate. The solution was filtered and concentrated in vacuo to give a brown solid. The solid was purified by flash column chromatography (EtOAc/CH2Cl2; 0:1, 1:9, 2:8, 3:7, 4:6) to give a white solid. The solid was suspended in methanol and acidified to pH 1 with HCl(g) and the resulting suspension was filtered. The filtrand was diluted with EtOAc to give a white precipitate which was collected by vacuum filtration to give 1a (800 mg, 34percent) that was used without further purification. 1H NMR δ (400 MHz, d6-DMSO) 8.54 (1H, s, H-6), 7.68 (1H, d, J 1.9, H-8), 7.05 (2H, m, H-3′, H-5′), 6.67-6.48 (3H, m, H-2′, H-4′, H-6′), 5.84 (1H, NH, br), 5.64 (1H, s, H-3), 5.16 (1H, q , J 6.7 Hz, CH3HNHAr), 3.77-3.52 (8H, m, morph), 2.71-2.62 (1H, m, H-Ar), 2.25 (3H, d, J 0.8, CH3), 1.49 (3H, d, J 6.7, HCH3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping