|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20℃; for 2h; |
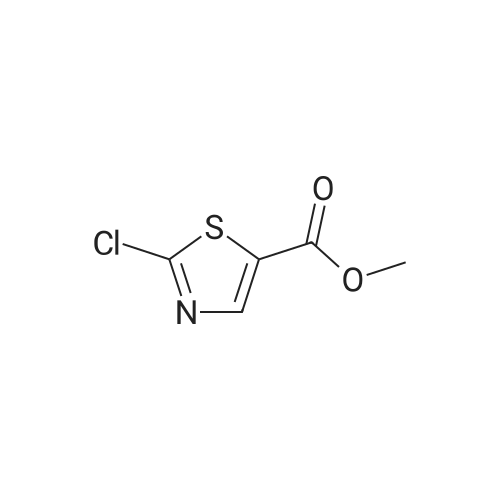
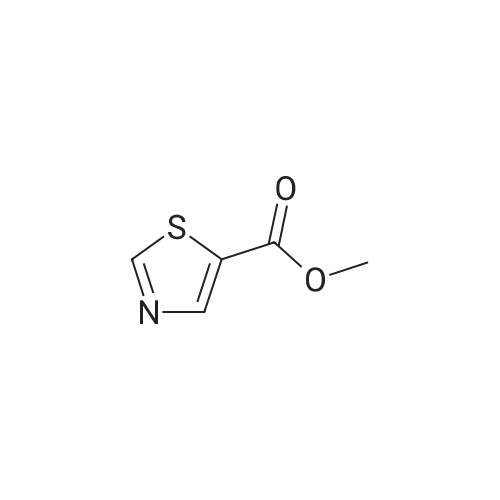
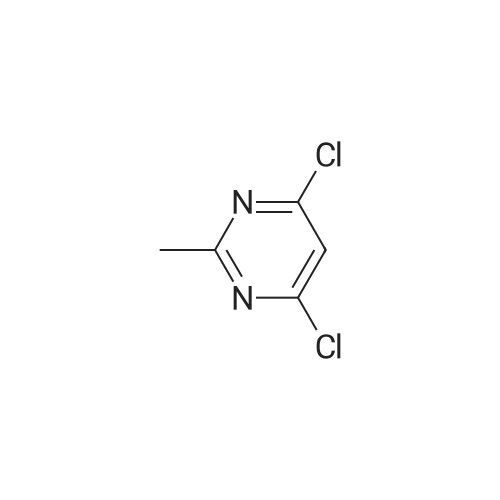
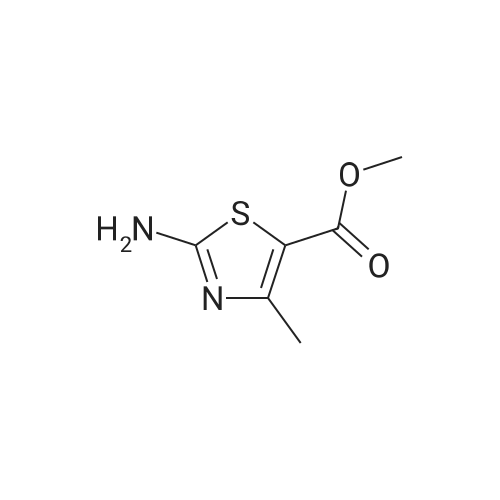
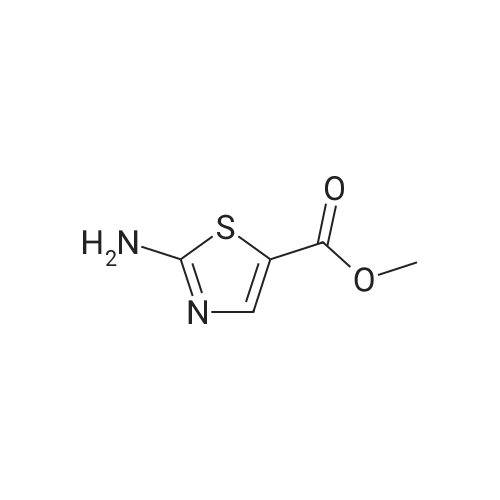
To a suspension of <strong>[6633-61-0]methyl 2-aminothiazole-5-carboxylate</strong> (4.90 g, 31.0 mmol) and NaH (60% dispersion in mineral oil, 1.36 g, 34.1 mmol) in DMF at O0C is added 4,6-dichloro-2-methyl-pyrimidine (5.05 g, 31.0 mmol) in DMF and the mixture is stirred for 2 hours at room temperature. The reaction mixture is diluted with EtOAc and washed with 10% aqueous sodium thiosulfate solution. The organic layer is dried over MgSO4, and concentrated in reduced pressure. The crude product is crystallized from MeOH to give methyl 2-(6-chloro-2-methyl-pyrimidin-4-ylamino)-thiazole-5-carboxylate as a white solid. [0065] To a stirred solution of methyl 2-(6-chloro-2-methyl-pyrimidin-4- ylamino)-thiazole-5-carboxylate (3.97 g, 14.0 mmol) in MeOH is added 4 N NaOH (15 mL) and the mixture is stirred for 12 hours at 6O0C. The reaction mixture is neutralized with 1 N HCl and the resulting precipitate is filtered and washed with MeOH to give 2-(6-chloro-2- methyl-pyrimidin-4-ylamino)-thiazole-5-carboxylic acid in a white solid. [0066] To a solution of 2-(6-chloro-2-methyl-pyrimidin-4-ylamino)-thiazole-5- carboxylic acid (230 mg, 0.85 mmol), N-(3-Amino-4-methyl-phenyl)-3- trifluoromethylbenzamide (250 mg, 0.85 mmol), and diisopropylethylamine (0.59 mL, 3.4 mmol) in DMF is added O-(7-azabenzotriazol-l-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (355 mg, 0.93 mmol), and the mixture is stirred for 12 hours at room temperature. The reaction mixture is diluted with EtOAc and washed with 10% aqueous sodium thiosulfate solution. The organic layer is dried over MgSO4 and concentrated in reduced pressure. The crude product is purified by preparative HPLC to give 2-(6-chloro-2- methyl-pyrimidin-4-ylamino)-thiazole-5-carboxylic acid [2-methyl-5-(3-trifluoromethyl- benzoylamino)-phenyl] -amide as a white solid.[0067] To a stirred solution of 2-(6-chloro-2-methyl-pyrimidin-4-ylamino)- thiazole-5-carboxylic acid [2-methyl-5-(3-trifluoromethyl-benzoylamino)-phenyl]-amide (25 EPO <DP n="27"/>mg, 46 mumol) in l,3-dimethyl-2-imidazolidinone (0.2 mL) is added excess 2-piperazin-l-yl- ethanol (100 mg) in l,3-dimethyl-2-imidazolidinone (0.2 mL) and the mixture is stirred for 4 hours at 60 C . The crude product is diluted with DMSO (1 mL) and purified by preparative HPLC to give 2-{6-[4-(2-Hydroxyethyl)-piperazin-l-yl]-2-methylpyrimidin-4-ylamino}- thiazole-5-carboxylic acid [2-methyl-5-(3-trifluoromethylbenzoylamino)-phenyl]-amide in a TFA salt form: 1H NMR 400 MHz (MeOH-d_0 delta 8.26 (s, IH), 8.20 (d, IH), 8.15 (s, IH), 7.90 (d, IH), 7.83 (s, IH), 7.74 (t, IH), 7.55 (d, IH), 7.31 (d, IH), 6.20 (br, IH), 3.93 (dd, 2H), 3.50 (br, 8H), 3.35 (dd, 2H), 2.53 (s, 3H), 2.31 (s, 3H); MS m/z 641.5(M + 1). |
| 62.89 g |
With sodium hydride; In tetrahydrofuran; N,N-dimethyl acetamide; at -5℃; for 3h; |
In a reaction flask, 49.49 g 4,6-dichloro-2-methylpyrimidine (0.303 mol), 40.00 g methyl 2-aminothiazol-5-carboxylic acid (0.253 mol), and 200 ml N,N-dimethylacetamide were charged, the temperature was brought to -5 C. and 18.20 g sodium hydride (0.455 mol) in 90 ml tetrahydrofuran were added dropwise and the reaction mixture was kept under these conditions for about three hours. At the end of the reaction, 250 ml of a solution of hydrochloric acid 2N were added, the temperature was brought to the room value, the formed solid was filtered and washed with water (4×200 ml) and dried in oven under vacuum at a temperature of 55 C. for about eight hours, to give 62.89 g methyl 2-(6-chloro-2-methylpyrimidin-4-yl-amino)thiazol-5-carboxylic acid.1H-NMR (DMSO, 300 MHz): delta 8.13 (1H, s), 6.97 (1H, s), 3.82 (3H, s), 2.59 (3H, s) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping