Alternatived Products of [ 65934-74-9 ]
Product Details of [ 65934-74-9 ]
| CAS No. : | 65934-74-9 |
MDL No. : | MFCD01631582 |
| Formula : |
C8H8F3N
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | JBCDCYFEJQHTTA-UHFFFAOYSA-N |
| M.W : |
175.15
|
Pubchem ID : | 2737715 |
| Synonyms : |
|
Application In Synthesis of [ 65934-74-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 65934-74-9 ]
- 1
-
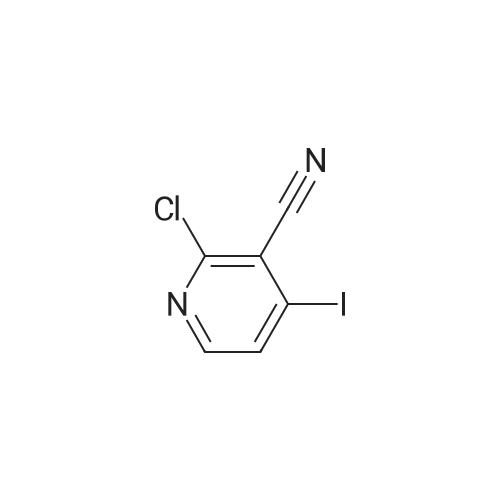 [ 1171919-75-7 ]
[ 1171919-75-7 ]

-
tert-butyl (3R)-3-[(2-sulfanylacetyl)amino]piperidine-1-carboxylate
[ No CAS ]

-
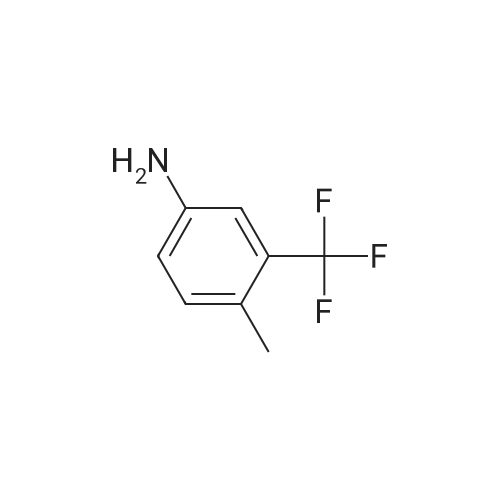 [ 65934-74-9 ]
[ 65934-74-9 ]

-
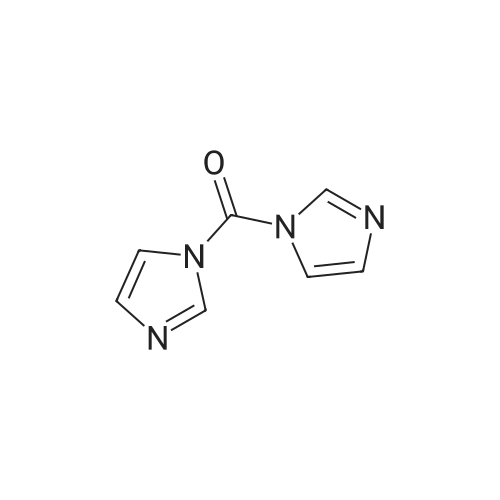 [ 530-62-1 ]
[ 530-62-1 ]

-
(R)-tert-butyl 3-(5-(4-methyl-3-(trifluoromethyl)phenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8-triazaacenaphthylene-2-carboxamido)piperidine-1-carboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 52.8% |
|
To a 2-5 mL Biotage microwave vial with a stir bar were added 4-methyl-3-(trifluoromethyl)aniline (80 mg, 0.41 mmol), <strong>[1171919-75-7]2-chloro-4-iodonicotinonitrile</strong> (107 mg, 0.405 mmol), Pd(OAc)2(1.8 mg, 0.0080 mmol), DPEPhos (6.8 mg, 0.013 mmol), and Cs2C03(184 mg, 0.565 mmol). The vial was sealed, treated with dioxane (0.81 mL), evacuated and flushed with argon 4X, and stirred at 150 C under argon for 30 min. The reaction was cooled to room temperature and was treated with tot- butyl (3R)-3-[(2-sulfanylacetyl)amino]piperidine-1-carboxylate (Intermediate 22) ( 0.49 M in dioxane, 0.83 mL, 0.41 mmol) via syringe. The vial was sealed and evacuated and flushed with argon 4X, and stirred at 150 C for 15 min. The reaction was then cooled to room temperature, treated with solid CDI (265 mg, 1.63 mmol) in one portion under air, resealed and evacuated and flushed with argon 4X, and stirred at 150 C for 15 min. The reaction was diluted with EtOAc (10 mL), and washed with 0.5 M citric acid and brine (2 x 8 mL) and 2 M K2C03(1 x 5 mL). The organic phase was dried over anhydrous Na2SC>4, filtered, and concentrated to dryness. The residue was purified by normal phase flash column chromatography (Si02) to give the title compound as a light-beige solid (123 mg, 52.8% yield). |
| 52.8% |
|
To a 2-5 mL Biotage microwave vial with a stir bar were added 4-methyl-3- (trifluoromethyl) aniline (80 mg, 0.41 mmol) , <strong>[1171919-75-7]2-chloro-4-iodonicotinonitrile</strong> (107 mg, 0.405 mmol) , Pd (OAc)2(1.8 mg, 0.0080 mmol) , DPEPhos (6.8 mg, 0.013 mmol) , and Cs2CO3(184 mg, 0.565 mmol) . The vial was sealed, treated with dioxane (0.81 mL) , evacuated and flushed with argon 4X, and stirred at 150 under argon for 30 min. The reaction was cooled to room temperature and was treated with tert-butyl (3R) -3- [ (2-sulfanylacetyl) amino] piperidine-1-carboxylate (Intermediate 22) (0.49 M in dioxane, 0.83 mL, 0.41 mmol) via syringe. The vial was sealed and evacuated and flushed with argon 4X, and stirred at 150 for 15 min. The reaction was then cooled to room temperature, treated with solid CDI (265 mg, 1.63 mmol) in one portion under air, resealed and evacuated and flushed with argon 4X, and stirred at 150 for 15 min. The reaction was diluted with EtOAc (10 mL) , and washed with 0.5 M citric acid and brine (2 x 8 mL) and 2 M K2CO3(1 x 5 mL) . The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated to dryness. The residue was purified by normal phase flash column chromatography (SiO2) to give the title compound as a light-beige solid (123 mg, 52.8yield) . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







