| 65% |
With hydrogenchloride; at 120 - 132℃; for 5h;Large scale; |
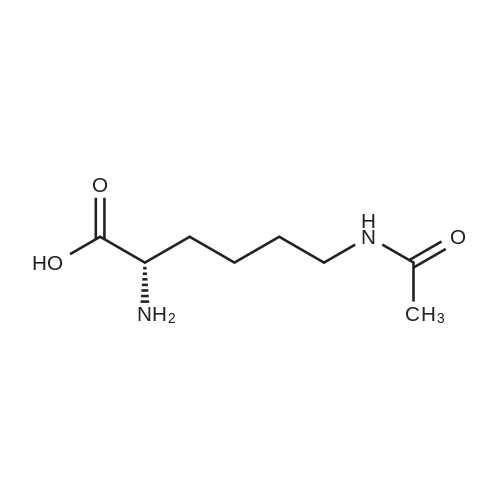
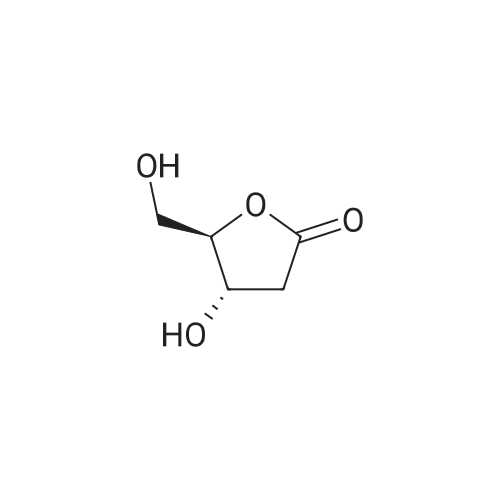
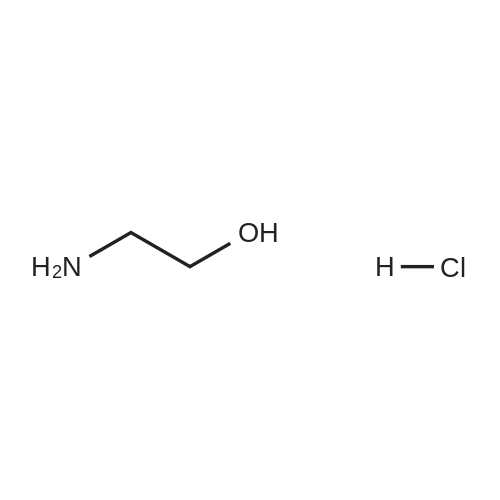
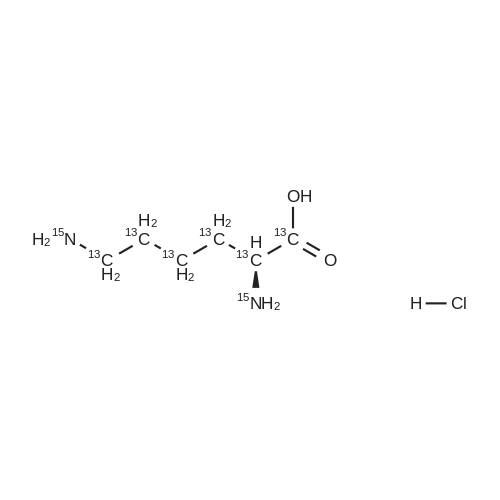
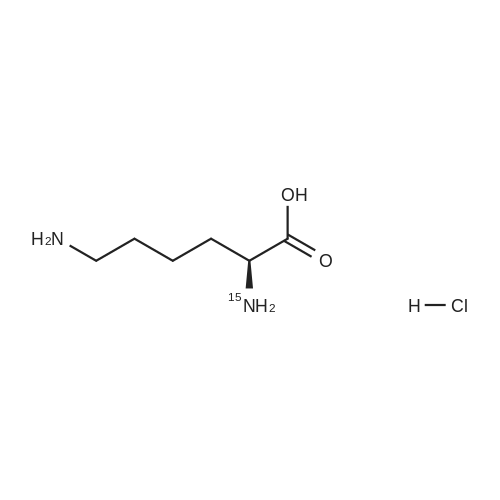
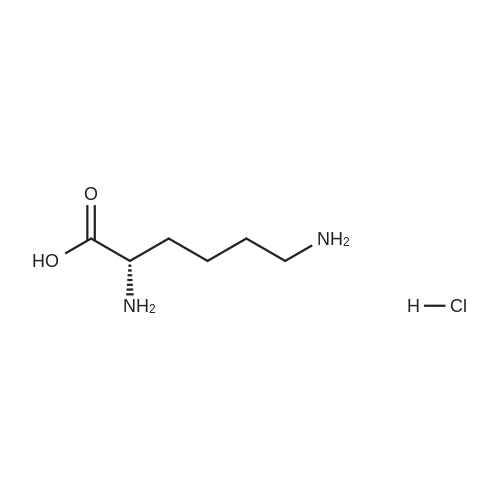
Example 1 Lysine Ester Trihydrochloride Salt Production Ethanolamine hydrochloride (1240 grams, 12.6 moles) was placed into a resin kettle fitted with mechanical stirrer, thermocouple, gas inlet tube and vacuum fitting. The solids were heated to approximately 90 C., where a melt was formed. Lysine mono-hydrochloride (1010 grams, 5.5 moles) was added in portions to the melt so as to maintain a free-flowing slurry. After the addition was complete, a vacuum (water aspirator) was established over the reaction mixture and the temperature was increased to 120 C. At the same time, HCl gas was bubbled into the reaction mixture. The rate was not measured but was estimated as 50-100 ml/min over a total addition time of about 5 hours. A significant exotherm was observed, reaching a maximum temperature of 132 C. The reaction mixture became a progressively thinner suspension as the temperature rose and eventually became a viscous, clear honey-colored oil. Once the reaction was complete (disappearance of lysine by 1H NMR), the mixture was cooled to 90 C. and cautiously diluted with methanol (5 liters) to give a solution. During the addition of methanol the solution cooled to the reflux temperature. This hot solution was further diluted with denatured ethanol (SDA 2B-4) to a total volume of approximately 17 liters (approximately 30 volume percent methanol). Solids formed on slow cooling overnight, which were isolated by vacuum filtration. The solids were deliquescent and had to be protected from exposure to air to avoid picking up moisture. The recovery was 1420 grams (86% yield). The trihydrochloride salt was purified by dissolving in methanol and subsequent dilution with ethanol near reflux temperature using the same ratios and loadings described above. In this manner, the product was isolated as a white, crystalline solid. Mass recovery was 1040 grams (65% yield). Additional product formed in the mother liquors over time, but was not recovered. FIG. 1 is a graphic illustration of the 1H NMR data obtained from isolated and purified lysine ester trihydrochloride salt. The lysine ester trihydrochloride salt had high purity (e.g., greater than 98%). |
| 65% |
With hydrogenchloride; at 120 - 132℃; for 5h;Large scale; |
Example 1 Lysine Ester Trihydrochloride Salt Production Ethanolamine hydrochloride (1240 grams, 12.6 moles) was placed into a resin kettle fitted with mechanical stirrer, thermocouple, gas inlet tube and vacuum fitting. The solids were heated to approximately 90 C., where a melt was formed. Lysine mono-hydrochloride (1010 grams, 5.5 moles) was added in portions to the melt so as to maintain a free-flowing slurry. After the addition was complete, a vacuum (water aspirator) was established over the reaction mixture and the temperature was increased to 120 C. At the same time, HCl gas was bubbled into the reaction mixture. The rate was not measured but was estimated as 50-100 ml/min over a total addition time of about 5 hours. A significant exotherm was observed, reaching a maximum temperature of 132 C. The reaction mixture became a progressively thinner suspension as the temperature rose and eventually became a viscous, clear honey-colored oil. Once the reaction was complete (disappearance of lysine by 1H NMR), the mixture was cooled to 90 C. and cautiously diluted with methanol (5 liters) to give a solution. During the addition of methanol the solution cooled to the reflux temperature. This hot solution was further diluted with denatured ethanol (SDA 2B-4) to a total volume of approximately 17 liters (approximately 30 volume percent methanol). Solids formed on slow cooling overnight, which were isolated by vacuum filtration. The solids were deliquescent and had to be protected from exposure to air to avoid picking up moisture. The recovery was 1420 grams (86% yield). The trihydrochloride salt was purified by dissolving in methanol and subsequent dilution with ethanol near reflux temperature using the same ratios and loadings described above. In this manner, the product was isolated as a white, crystalline solid. Mass recovery was 1040 grams (65% yield). Additional product formed in the mother liquors over time, but was not recovered. FIG. 1 is a graphic illustration of the 1H NMR data obtained from isolated and purified lysine ester trihydrochloride salt. The lysine ester trihydrochloride salt had high purity (e.g., greater than 98%). |
| 65% |
With hydrogenchloride; In chlorobenzene; at 90 - 120℃; for 5h;Industrial scale; |
Ethanolamine hydrochloride (1240 grams, 12.6 moles) was placed into a resin kettle fitted with mechanical stirrer, thermocouple, gas inlet tube and vacuum fitting. The solids were heated to approximately 90 C., where a melt was formed. Lysine mono-hydrochloride (1010 grams, 5.5 moles) was added in portions to the melt so as to maintain a free-flowing slurry. After the addition was complete, a vacuum (water aspirator) was established over the reaction mixture and the temperature was increased to 120 C. At the same time, HCl gas was bubbled into the reaction mixture. The rate was not measured but was estimated as 50-100 ml/min over a total addition time of about 5 hours. A significant exotherm was observed, reaching a maximum temperature of 132 C. The reaction mixture became a progressively thinner suspension as the temperature rose and eventually became a viscous, clear honey-colored oil. Once the reaction was complete (disappearance of lysine by 1H NMR), the mixture was cooled to 90 C. and cautiously diluted with methanol (5 liters) to give a solution. During the addition of methanol the solution cooled to the reflux temperature. This hot solution was further diluted with denatured ethanol (SDA 2B-4) to a total volume of approximately 17 liters (approximately 30 volume percent methanol). Solids formed on slow cooling overnight, which were isolated by vacuum filtration. The solids were deliquescent and had to be protected from exposure to air to avoid picking up moisture. The recovery was 1420 grams (86% yield). The trihydrochloride salt was purified by dissolving in methanol and subsequent dilution with ethanol near reflux temperature using the same ratios and loadings described above. In this manner, the product was isolated as a white, crystalline solid. Mass recovery was 1040 grams (65% yield). Additional product formed in the mother liquors over time, but was not recovered. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping