|
With hydrogenchloride; thionyl chloride; ethanethiol;aluminium trichloride; In tetrahydrofuran; N-methyl-acetamide; dichloromethane; water; chlorobenzene; |
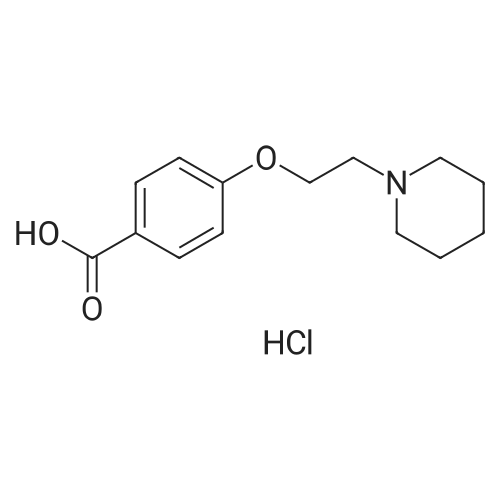
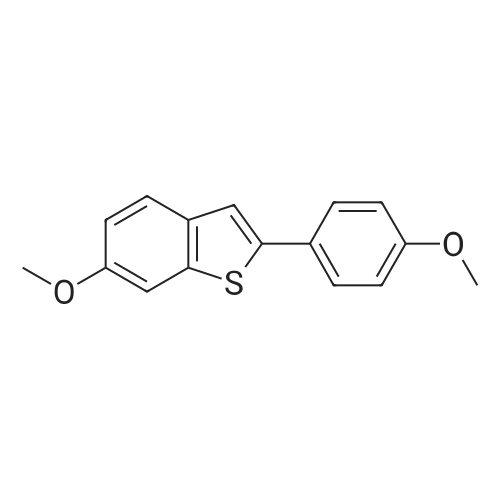
EXAMPLE 3 6-hydroxy-2-(4-hydroxyphenyl)-3[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene, hydrochloride Under a nitrogen blanket, a mixture of 3 g. of 4-(2-piperidinoethoxy) benzoic acid, hydrochloride, 2 drops of dimethylformamide, 2.5 ml. of thionyl chloride and 40 ml. of chlorobenzene was heated at 70°-75° C. for about one hour. The excess thionyl chloride and 15-20 ml. of solvent were then distilled off. The remaining suspension was cooled to ambient temperature, and to it were added 100 ml. of dichloromethane, 2.7 g. of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene and 10 g. of aluminum chloride. The solution was stirred for about one hour, 7.5 ml. of ethanethiol was added, and the mixture was stirred for 45 minutes more. Then 40 ml. of tetrahydrofuran was added, followed by 15 ml. of 20percent hydrochloric acid, with an exotherm to reflux. Fifty ml. of water and 25 ml. of saturated aqueous sodium chloride were added. The mixture was stirred and allowed to cool to ambient temperature. The precipitate was collected by filtration and washed successively with 30 ml of water, 40 ml of 25percent aqueous tetrahydrofuran, and 35 ml. of water. The solids were then dried at 40° C. under vacuum to obtain 5.05 g. of product, which was identified by nmr. delta1.7(6H, m, N(CH2 CH2)2 CH2); 2.6-3.1 (2H, m, NCH2); 3.5-4.1 (4H, m, NCH2); 4.4 (2H, m, OCH2); 6.6-7.4 (9H, m, aromatic); 7.7 (2H, d, aromatic o to CO); 9.8(2H, m, OH). |
|
With hydrogenchloride; thionyl chloride; ethanethiol;aluminium trichloride; In tetrahydrofuran; N-methyl-acetamide; dichloromethane; water; chlorobenzene; |
PREPARATION 3 6-hydroxy-2-(4-hydroxyphenyl)-3[-4-(2piperidinoethoxy)benzoyl]benzo[b]thiophene, hydrochloride Under a nitrogen blanket, a mixture of 3 g. of 4-(2piperidinoethoxy)benzoic acid, hydrochloride, 2 drops of dimethylformamide, 2.5 ml. of thionyl chloride and 40 ml. of chlorobenzene was heated at 70°-75° C. for about one hour. The excess thionyl chloride and 15-20 ml. of solvent were then distilled off. The remaining suspension was cooled to ambient temperature, and to it were added 100 ml. of dichloromethane, 2.7 g. of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene and 10 g. of aluminum chloride. The solution was stirred for about one hour, 7.5 ml. of ethanethiol was added, and the mixture was stirred for 45 minutes more. Then 40 ml. of tetrahydrofuran was added, followed by 15 ml. of 20percent hydrochloric acid, with an exotherm to reflux. Fifty ml. of water and 25 ml. of saturated aqueous sodium chloride were added. The mixture was stirred and allowed to cool to ambient temperature. The precipitate was collected by filtration and washed successively with 30 ml. of water, 40 ml of 25percent aqueous tetrahydrofuran, and 35 ml. of water. The solids were then dried at 40° C. under vacuum to obtain 5.05 g. of product, which was identified by nmr. delta1.7(6H, m, N(CH2 CH2)2 CH2); 2.6-3.1(2H, m, NCH2); 3.5-4.1 (4H, m, NCH2); 4.4(2H, m, OCH2); 6.6-7.4(9H, m, aromatic); 7.7(2H, d, aromatic o to CO); 9.8(2H, m, OH). |
|
With hydrogenchloride; thionyl chloride; ethanethiol;aluminium trichloride; In tetrahydrofuran; N-methyl-acetamide; dichloromethane; water; chlorobenzene; |
EXAMPLE 1 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene, hydrochloride Under a nitrogen blanket, a mixture of 3 g. of 4-(2-piperidinoethoxy)benzoic acid, hydrochloride, 2 drops of dimethylformamide, 2.5 ml. of thionyl chloride and 40 ml. of chlorobenzene was heated at 70°-75° C. for about one hour. The excess thionyl chloride and 15-20 ml. of solvent were then distilled off. The remaining suspension was cooled to ambient temperature, and to it were added 100 ml. of dichloromethane, 2.7 g. of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene and 10 g. of aluminum chloride. The solution was stirred for about one hour, 7.5 ml. of ethanethiol was added, and the mixture was stirred for 45 minutes more. Then 40 ml. of tetrahydrofuran was added, followed by 15 ml. of 20percent hydrochloric acid, with an exotherm to reflux. Fifty ml. of water and 25 ml. of saturated aqueous sodium chloride were added. The mixture was stirred and allowed to cool to ambient temperature. The precipitate was collected by filtration and washed successively with 30 ml. of water, 40 ml. of 25percent aqueous tetrahydrofuran, and 35 ml. of water. The solids were then dried at 40° C. under vacuum to obtain 5.05 g. of product, which was identified by its nmr spectrum, using a 90 mHz instrument and deuterochloroform. delta1.7 (6H, m, N(CH2 CH2)2 CH2); 2.6-3.1 (2H, m, NCH2); 3.5-4.1 (4H, m, NCH2); 4.4 (2H, m, OCH2); 6.6-7.4 (9H, m, aromatic); 7.7(2H, d, aromatic o to CO); 9.8 (2H, m, OH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping