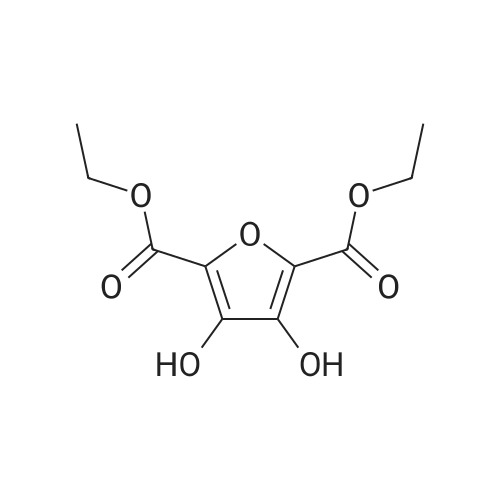
|
With oxygen; sodium carbonate; In water; at 20 - 50℃; for 2h; |
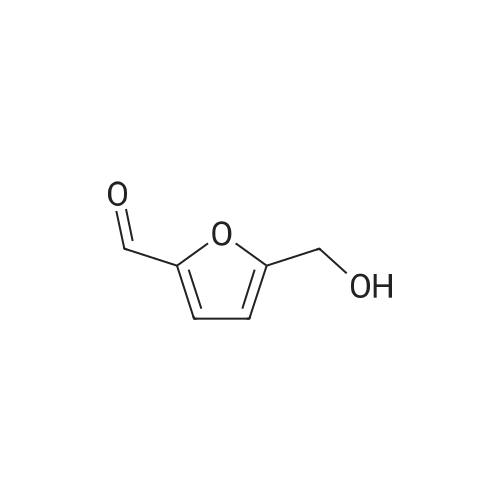
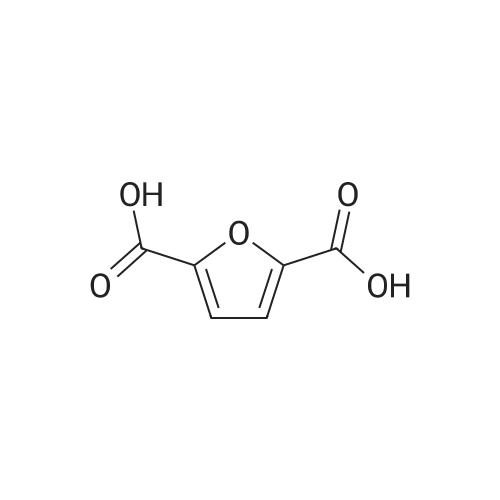
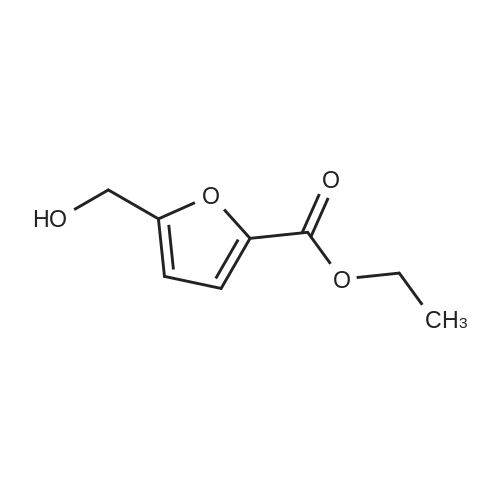
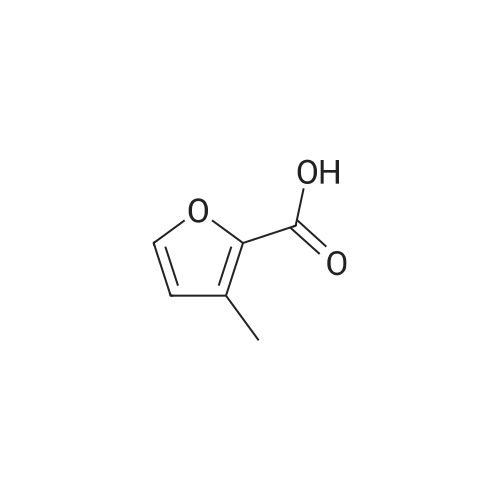
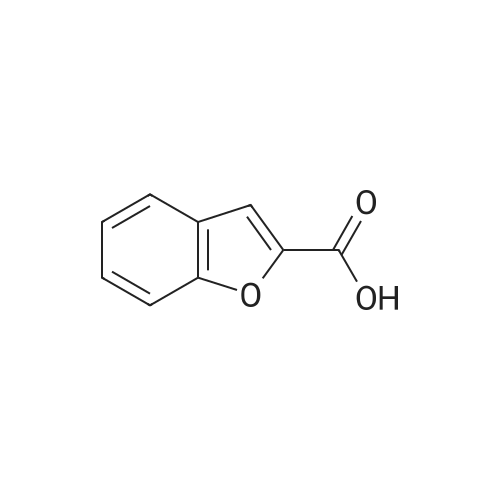
Catalytic Reaction Of HMF To FDCA For this reaction, Na2C03 was used as the base. 1 g of extracted HMF was first dissolved in 5 g of water. The Na2C03 was separately prepared by dissolving Na2C03 in water. The oxidation catalyst was then added follow by the HMF solution at ambient room temperature. With oxygen gas bubbling, the solution was first heated to 50C for 2 hours, and HMF was fully converted to HFCA. After that, the reaction was heat to 95C and kept for 7 hour. The pH of the aqueous solution was then adjusted to 1 and FDCA was precipitated from the solution. The precipitate was filtered and washed with ethanol. |
| 2.74 g |
With 4% Au/TiO2; oxygen; sodium hydroxide; In water; at 70℃; for 7h;pH 10; |
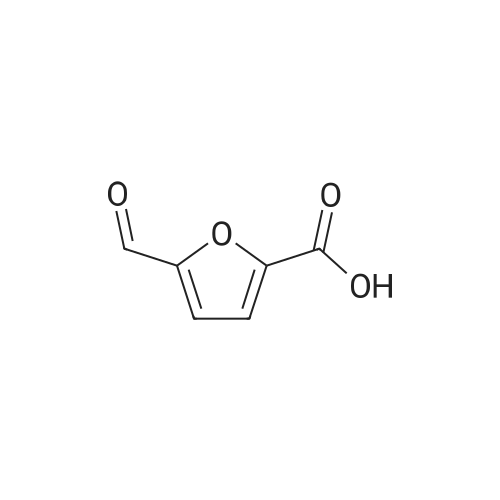
10 g of HMF, 150 g of water, and 1 g of catalyst (4% Au/TiO2) were added into a round bottom bottle (250 mL) and then heated to 70 C. Air under atmosphere pressure was introduced into the liquid in the flask. The pH value of the above reaction was controlled to 10 by adding a sodium hydroxide aqueous solution into the flask. The reaction was continued for 7 hours to obtain a crude aqueous solution. The crude aqueous solution was extracted by 200 mL of ethyl acetate two times, and the aqueous phase of the extractions was collected by a separatory funnel. The collected aqueous phase was titrated by concentrated hydrochloric acid (HCl) until its pH value reached 3. The acidified aqueous phase was extracted by 200 mL of ethyl acetate two times, and the organic phase of the extractions was collected. The collected organic phase was vacuumed concentrated to obtain 2.74 g of solid, which was 5-hydroxymethyl-2-furoic acid (HMFCA). The above reaction is shown in Formula 8. The product of Formula 8 had NMR spectra as below: 1H NMR (400 MHz, d-DMSO): 13.08 (br, 1H), 7.14 (d, 1H, J=3.4 Hz), 6.45 (d, 1H, J=3.4 Hz), 5.59 (s, 1H), 4.44 (s, 2H); 13C NMR (100 M Hz, d-DMSO): 160.1, 159.8, 144.4, 119.0, 109.4, 56.2. |
| 2.74g |
With gold on titanium oxide; oxygen; sodium hydroxide; In water; at 70℃; under 760.051 Torr; for 7h;pH 10; |
10 g of HMF,150 g of water,And 1 g of 4% Au / TiO2 catalyst250 mL of a round bottom flask,Heated to 70 C,And air was introduced under normal pressure.The pH of the reaction was then controlled to 10 with the addition of aqueous sodium hydroxide,After 7 hours of continuous reaction, an aqueous solution of the crude product was obtained.The crude aqueous solution was added to 200 mL of ethyl acetate for extraction twice,The water layer was taken in a separatory funnel.The aqueous layer was titrated with concentrated hydrochloric acid (HCl) until the pH was 3.After extraction twice with 200 mL of ethyl acetate, the organic layer was taken.The organic layer was concentrated under reduced pressure to give 2.74 g of a solid,5-hydroxymethyl-2-furoic acid (HMFCA).The above reaction is shown in Formula 8 below. |
|
With sodium hydroxide; In water; at 30℃; under 2250.23 Torr; for 5h; |
The Au / MgO (Au0.5 wt%) catalyst, 1 mmol of 5-hydroxymethylfurfural, NaOH, 10 ml of water was charged to a stainless steel autoclave with a Teflon-lined internal metal, 5-hydroxymethylfurfural: NaOH = 0.015: 1: 4 (mol: mol: mol).Using automatic temperature control program temperature to the reaction temperature of 30 C, adding 0.3MPa oxygen, reaction 5 hours, the reaction process to maintain the same pressure.The reaction product was analyzed by HPLC. |
| 95%Chromat. |
With recombinant Escherichia coli cells expressing 3-succinoylsemialdehyde-pyridine dehydrogenase from Comamonastestosteroni SC1588; In aq. phosphate buffer; at 30℃; for 5h;pH 7;Enzymatic reaction; |
General procedure: Typically, 4 mL of phosphate buffer (0.2 M, pH 7) containing 50mMFF and 50 mg (cell wet weight) per mL microbial cells was incubated at30 C and 160 r/min. Aliquots were withdrawn from the reaction mixturesat specified time intervals and diluted with the correspondingmobile phase prior to HPLC analysis. The conversion was defined as theratio of the consumed substrate amount to the initial substrate amount(in mol). The yield was defined as the ratio of the formed productamount to the theoretical value based on the initial substrate amount(in mol). The selectivity was defined as the ratio of the formed productamount to the total amount of all products (in mol). All the experimentswere conducted at least in duplicate, and the values were expressed asthe means ± standard deviations. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping