| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
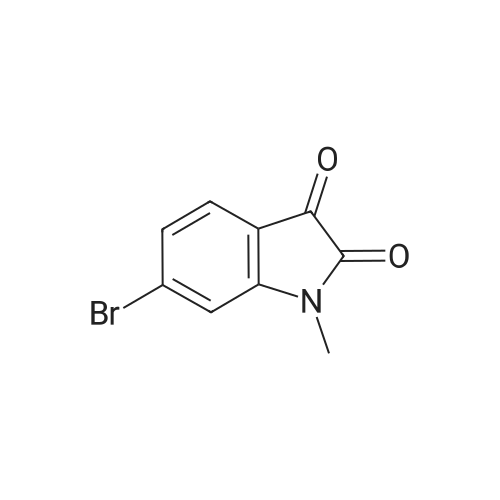
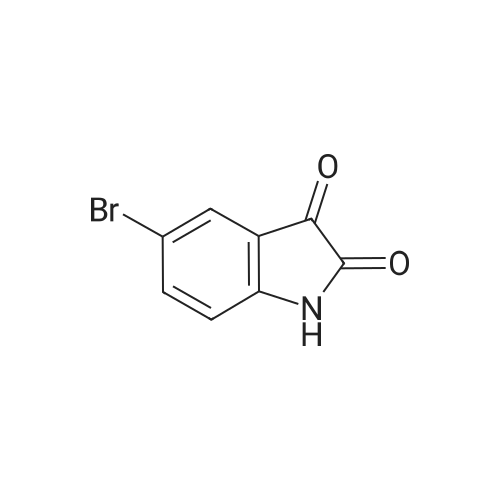
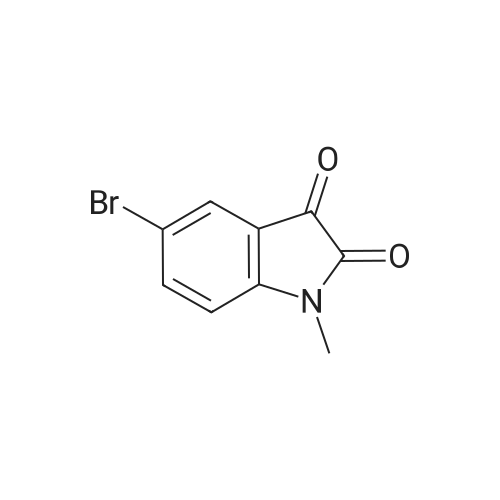
To R7 (25.0 g, I I I mmol) in acetonitrile (750 mL) is added Mel (15 mL, 24ltnmol) and KzC03(60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C for 2 h. The reaction mixture isfiltered and concentrated. Water and ethyl acetate are added to the residue. The organic layer isextracted twice with water, dried over MgS04 and concentrated. Yield 56%, mlz 240/242 [M+H]+,rt 0.48 min, LC-MS Method XOOl_ 004. |
| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
Step 1: Synthesis of Intermediate 1-5.1To RiO (25.0 g, 111 mmol) in acetonitrile (750 mL) is added Mel (15 mL, 241 mmol) and K2C03(60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C for 2 h. The reaction mixture isfiltered and concentrated. Water and ethyl acetate are added to the residue. The organic layer isextracted twice with water, is dried over MgSO4 and concentrated. Yield 56%, m/z 240/242[M+H]+, rt 0.48 mi LC-MS Method X001 004. |
| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
To R7 (25.0 g, 111 mmol) in acetonitrile (750 mL) is added MeI (15 mL, 241 mmol) and K2CO3 (60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C. for 2 h. The reaction mixture is filtered and concentrated. Water and ethyl acetate are added to the residue. The organic layer is extracted twice with water, dried over MgSO4 and concentrated. Yield 56%, m/z 240/242 [M+H]+, rt 0.48 min, LC-MS Method X001-004. |
| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
Synthesis of 1-Methyl-6-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1,3-dihydro-indol-2-one (R7) Step 1: Synthesis of Intermediate I-4.1 [0293] To R12 (25.0 g, 111 mmol) in acetonitrile (750 mL) is added MeI (15 mL, 241 mmol) and K2CO3 (60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C. for 2 h. The reaction mixture is filtered and concentrated. Water and ethyl acetate are added to the residue. The organic layer is extracted twice with water, dried over MgSO4 and concentrated. Yield 56%, m/z 240/242 [M+H]+, rt 0.48 min, LC-MS Method X001-004. |
| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
To R12 (25.0 g, 111 mmol) in acetonitrile (750 mL) is added Mel (15 niL, 241 mmol) and K2C03 (60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C for 2 h. The reaction mixture is filtered and concentrated. Water and ethyl acetate are added to the residue. The organic layer is extracted twice with water, dried over MgS04 and concentrated. Yield 56%, m/z 240/242 [M+H]+, rt 0.48 min, LC-MS Method X001 004. |
| 56% |
With potassium carbonate; In acetonitrile; at 60℃; for 2h; |
To R27 (25.0 g, 111 mmol) in acetonitrile (750 mL) is added MeI (15 mL, 241 mmol) and K2CO3 (60.0 g, 434 mmol) and the reaction mixture is stirred at 60 C. for 2 h. The reaction mixture is filtered and concentrated. Water and ethyl acetate are added to the residue. The organic layer is extracted twice with water, dried over MgSO4 and concentrated. Yield 56%, m/z 240/242 [M+H]+, rt 0.48 min, LC-MS Method X001-004. |
|
With potassium carbonate; In acetonitrile; at 60℃; for 0.666667h; |
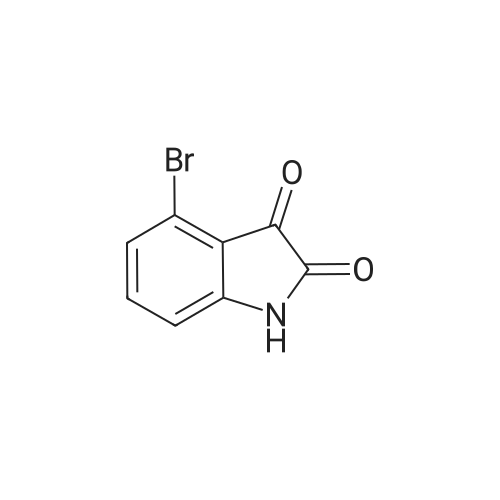
To a solution of 6-bromorsatin (CASNo. 6326-79-0, 4 52 g, 20 0 mmol) in acetonrtrile (150 mL) was added potassium carbonate (11 1 g, 80 mmol) followed by iodomethane (2 75 mL, 44 0 mmol) The reactron was then placed at 60 C and stirred for 40 minutes The reaction was then cooled to room temperature, tittered and concentrated to 10% of the original volume The reaction was then diluted with dichloromethane, water and brine The layers were separated and the aqueous layer was extracted two additional times with dichloromethane. The organic extracts were combined, dried over anhydrous sodium sulfate filtered and concentrated to provide 6-bromo-1-methyl-1 H-iotandole-2,3- dione as an orange solid without the need for further punfication The beta-bromo-1-methyl- 1 H-iotandole-2,3-diotaone (1 0 g, 4 2 mmol) was then treated with hydrazine hydrate (7 0 mL, 225 mmol) The reaction was heated to 130 C and stirred for 80 minutes, at which time the reaction was placed at room temperature and cooled by the addition of ice Once the reaction was cooled to room temperature it was diluted with dichloromethane and water and the layers were separated The aqueous layer was extracted an additional two times with dichloromethane, and the organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated The resulting residue was purified by silica gel flash chromatography (ethanol-dichloromethane 0 to 2%) to afford 6-bromo- 1-methyl-1 ,3-diotahydro-mdol-2-one, MS (ES+) m/z 225 9 (IvH-H)* |
|
|
General procedure: Isatins I (3.0 mmol) was dissolved in anhydrous DMF (15 mL), and the resultant solution was cooled to 0 C (ice bath), whereupon sodium hydride (60% dispersion in mineral oil, 140mg, 3.5 mmol, 1.17 equiv) was added in one portion and stirred for 5 minutes. Iodomethane (497 mg, 3.5 mmol, 1.17 equiv) or benzyl bromide (564.3 mg, 3.3 mmol, 1.1 equiv) was added and the reaction was stirred at 0 C for 30min. The reaction was monitored by TLC until I was fully consumed. The reaction mixture was then poured into saturated aqueous NH4Cl and extracted with ethyl acetate. The combined organic portions were washed with water and brine, dried (MgSO4), filtered, and concentrated to give N-methyl isatins and N-benzyl isatins II (90%-100% yield). |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0℃; |
General procedure: Step 1: To a 250 mL flask equipped with a silicone oil bubbler was added commercially available isatin (7.7 g, 50 mmol) and anhydrous DMF (80 mL). the mixture was cooled down to 0 oC. To this solution was added NaH (1.32 g, 55 mmol), followed by the addition of CH3I in 15 min. Upon completion of the reaction (monitored by TLC), the mixture was diluted with saturated NH4Cl solution and extracted with ethyl acetate. The organic layer was washed with water, dried over Na2SO4, filtered, and concentrated to yield the crude N-methylindoline-2, 3-dione, which was used directly in the next step. Step 2: The N-methylindoline-2, 3-dione (7.58 g, 47 mmol) was refluxed in NH2·NH2-H2O ( 35 %) for 1h. Then the mixture was cooled to rt. The crude product was extracted with ethyl acetate. The combined organic layer was then dried over Na2SO4, purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 10:1). 1-Methylindolin-2-one was obtained as a pink solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping