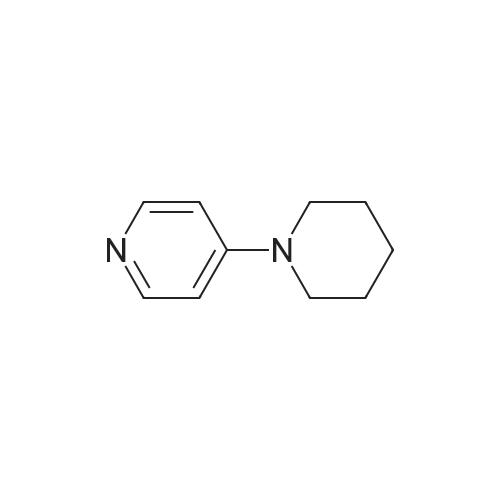
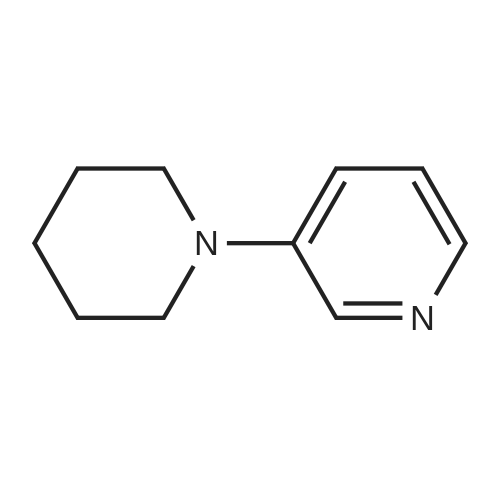
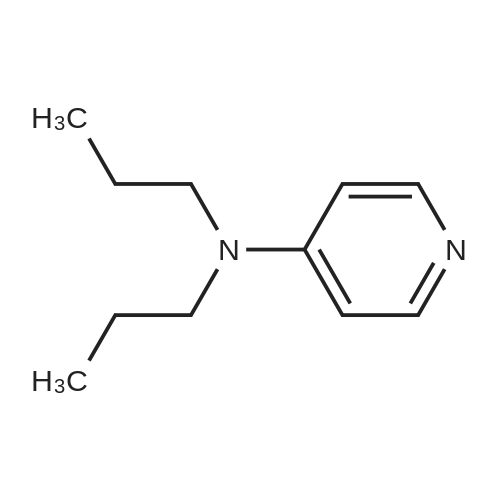
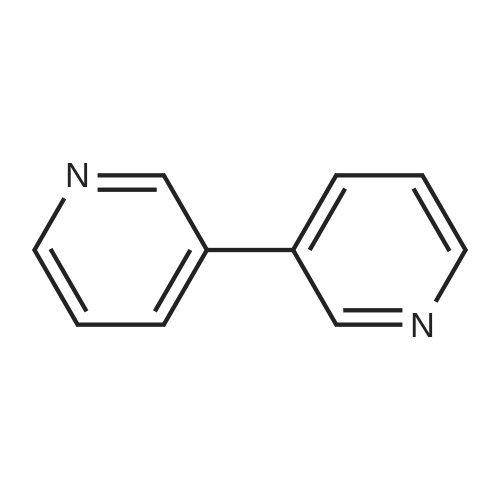
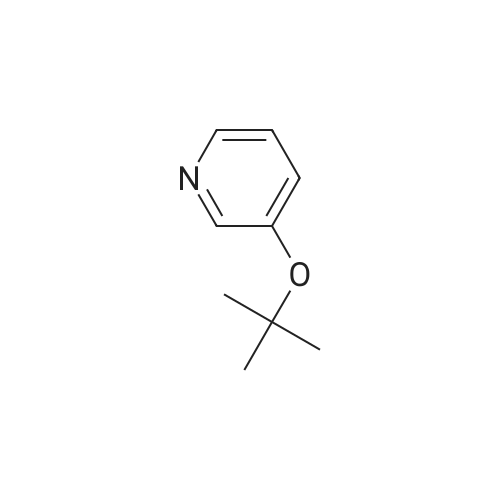
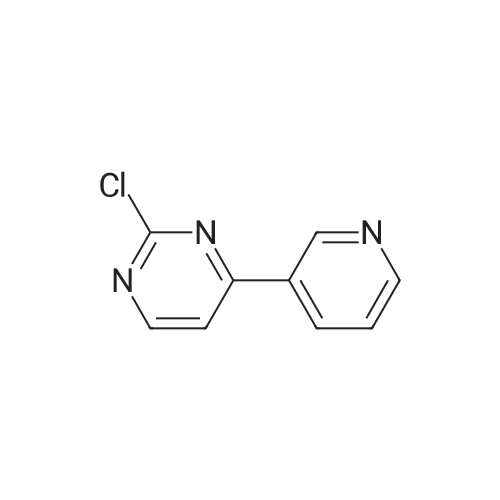
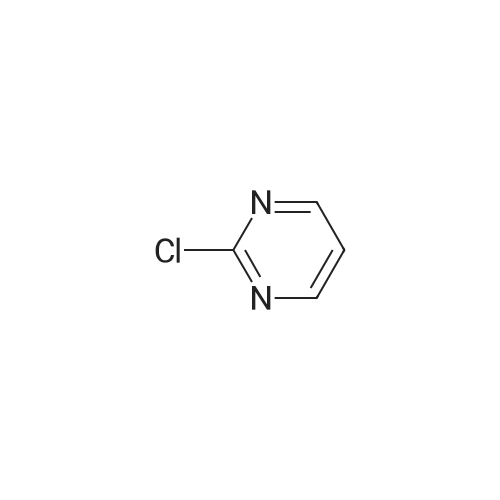
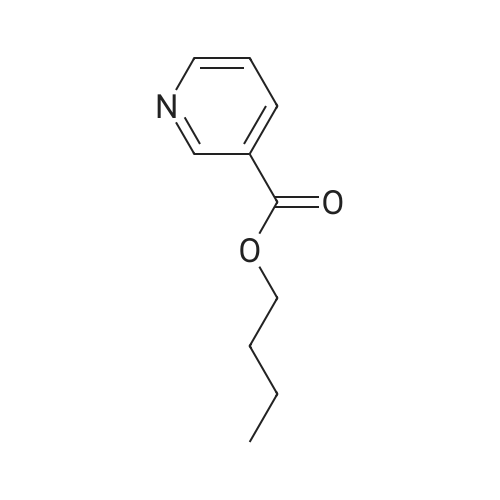
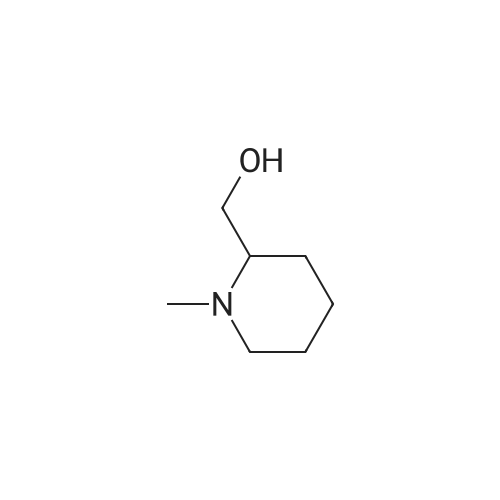
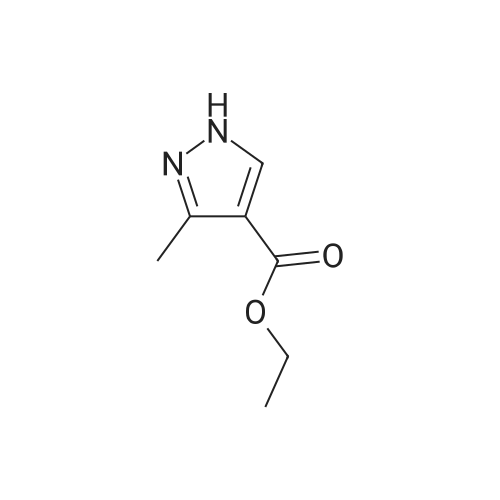
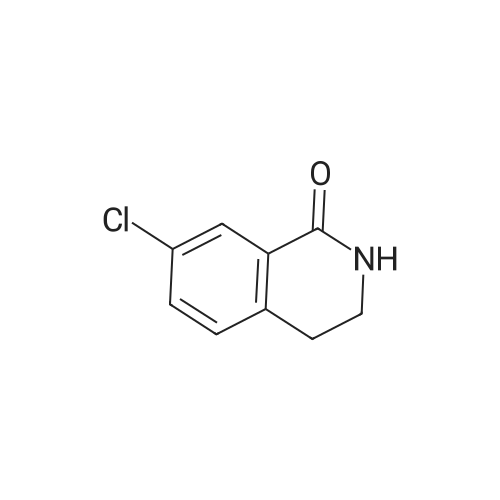
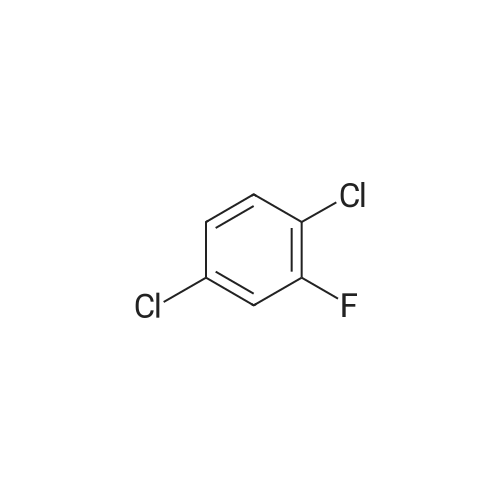
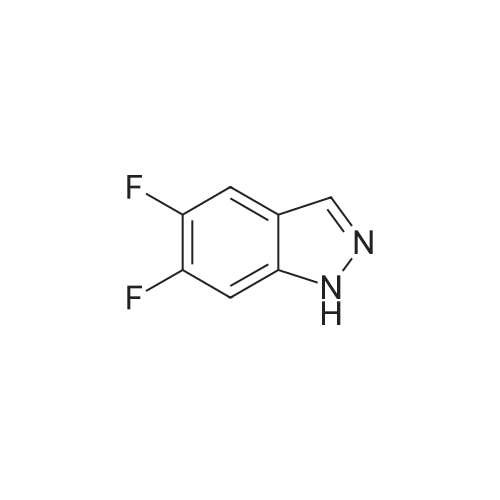
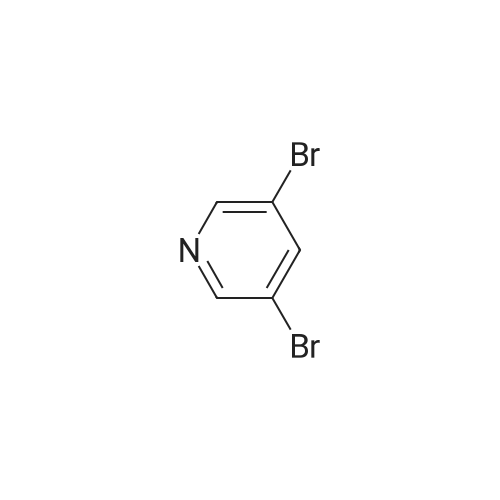
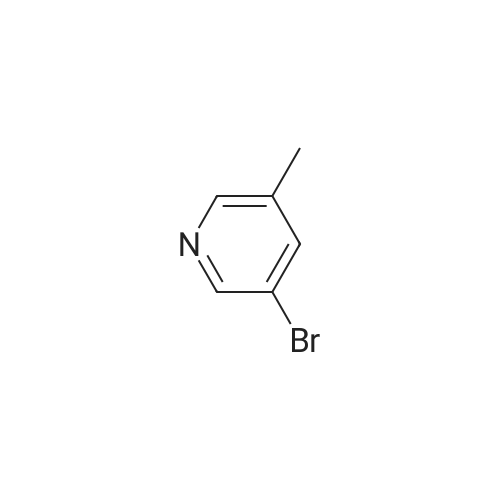
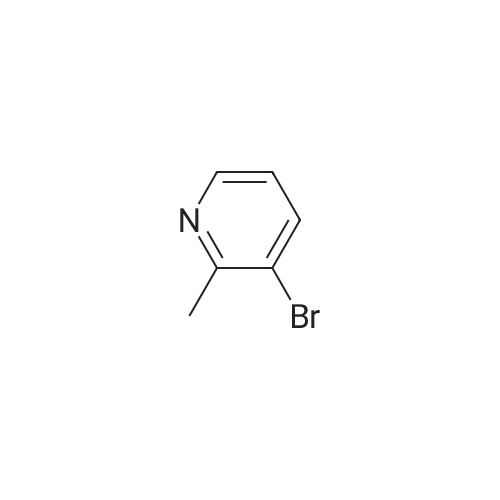
| 4% |
With oxalic acid; potassium carbonate; triphenylphosphine; copper(I) bromide; In ethanol; |
EXAMPLE 26 3-((1-methyl-2-piperidinyl)methoxy)pyridine oxalate salt 1-Methyl-2-piperidinemethanol (0.857 g, 6.65 mmol) was allowed to react with 3-bromopyridine (0.67 mL, 6.98), cuprous bromide (0.257 g, 1.33 mmol), triphenylphosphine (0.698 g, 2.66 mmol) and potassium carbonate (0.919 g, 6.65 mmol). The reaction mixture was heated to 90° C. and stirred for 120 hr, then cooled to 25° C., acidified with HCl (1.5 M; 35 mL) and washed with ethyl acetate (4*50 mL). The aqueous layer was basified with saturated aqueous potassium carbonate, and the product was extracted with chloroform (6*100 mL), dried (MgSO4) and concentrated in vacuo to an oil. The crude product was purified to yield the free base of the title compound after chromatography on silica gel (CHCl3 /MeOH/NH4 OH 1500:30:3). The amine was dissolved in EtOH (1 mL) and treated with oxalic acid (ca. 65 mg) to yield after recrystallization (EtOH/Et2 O) the title compound (0.088 g, 4percent) as a hygroscopic white solid. MS (DCI/NH3) m/e: 207 (M+H)+. 1 H NMR (D2 O,300 MHz) 5: 8.46 (d, J=2.9 Hz, 1H), 8.37 (dd, J=5.2, 1.1 Hz, 1H), 7.94 (ddd, J=8.8, 2.9, 1.1 Hz, 1H), 7.80 (dd, J=8.8, 5.9 Hz, 1H), 4.69 (dd, J=11.2, 3.1 Hz, 1H), 4.35 (dd, J=11.2, 2.0 Hz, 1H), 3.56 (m, 2H), 3.18 (dt, J=12.7, 3.0 Hz, 1H), 2.93 (s, 3H), 2.05-1.65 (m, 6H). Anal. calcd for C14 H20 N2 O5 Omega0.4 C2 H2 O4: C, 53.49; H, 6.31; N, 8.43. Found: C, 53.39; H, 6.09; N, 8.19. |
| 4% |
With oxalic acid; potassium carbonate; triphenylphosphine; copper(I) bromide; In ethanol; |
EXAMPLE 26 3-((1-methyl-2-piperidinyl)methoxy)pyridine oxalate salt 1-Methyl-2-piperidinemethanol (0.857 g, 6.65 mmol) was allowed to react with 3-bromopyridine (0.67 mL, 6.98), cuprous bromide (0.257 g, 1.33 mmol), triphenylphosphine (0.698 g, 2.66 mmol) and potassium carbonate (0.919 g, 6.65 mmol). The reaction mixture was heated to 90° C. and stirred for 120 hr, then cooled to 25° C., acidified with HCl (1.5 M; 35 mL) and washed with ethyl acetate (4*50 mL). The aqueous layer was basified with saturated aqueous potassium carbonate, and the product was extracted with chloroform (6*100 mL), dried (MgSO4) and concentrated in vacuo to an oil. The crude product was purified to yield the free base of the title compound after chromatography on silica gel (CHCl3 /MeOH/NH4 OH 1500:30:3). The amine was dissolved in EtOH (1 mL) and treated with oxalic acid (ca. 65 mg) to yield after recrystallization (EtOH/Et2 O) the title compound (0.088 g, 4percent) as a hygroscopic white solid. MS (DCI/NH3) m/e: 207 (M+H)+. 1 H NMR (D2 O, 300 MHz) delta: 8.46 (d, J=2.9 Hz, 1H), 8.37 (dd, J=5.2, 1.1 Hz, 1H), 7.94 (ddd, J=8.8, 2.9, 1.1 Hz, 1H), 7.80 (dd, J=8.8, 5.9 Hz, 1H), 4.69 (dd, J=11.2, 3.1 Hz, 1H), 4.35 (dd, J=11.2, 2.0 Hz, 1H), 3.56 (m, 2H), 3.18 (dt, J=12.7, 3.0 Hz, 1H), 2.93 (s, 3H), 2.05-1.65 (m, 6H). Anal. calcd for C14 H20 N2 O5.0.4 C2 H2 O4: C, 53.49; H, 6.31; N, 8.43. Found: C, 53.39; H, 6.09; N, 8.19. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping