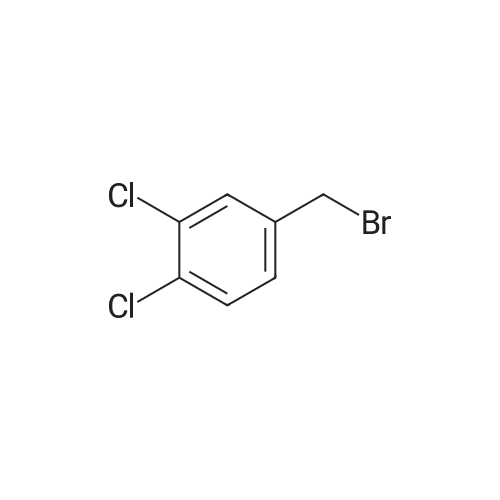
| 78% |
With potassium carbonate; In acetonitrile; for 7h;Reflux; |
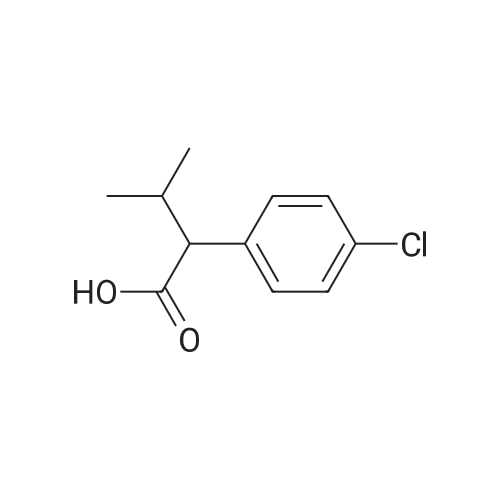
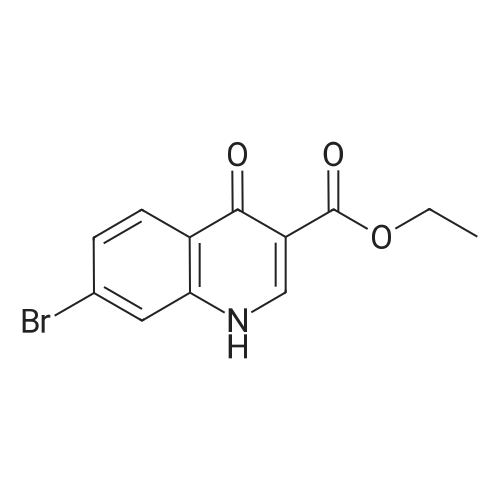
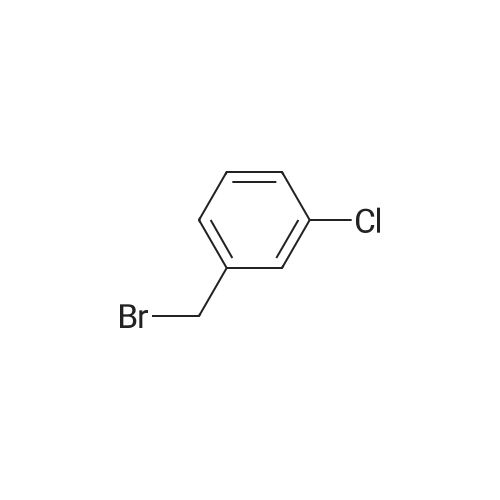
Step 1 Potassium carbonate (0.7 g, 5.1 mmol) and 4-chlorobenzyl bromide (0.76 g, 3.7 mmol) were added to mixture of 7-bromo-3-(ethoxycarbonyl)quinolin-4(1H)-one (1.0 g, 3.4 mmol) and acetonitrile (20 mL), and stirred under heating at reflux for 7 hours. The insoluble matter was removed by filtering, and the filtrate was washed by ethyl acetate and concentrated in vacuo. The resulting solid was washed by water and hexane to give 7-bromo-1-(4-chlorobenzyl)-3-(ethoxycarbonyl)quinolin-4(1H)-one (1.11 g, yield: 78%) as colorless solid. 1H-NMR (delta ppm TMS/DMSO-d6): 1.29 (3H, t, J=6.0 Hz), 4.24 (2H, q, J=6.0 Hz), 5.69 (2H, s), 7.28 (2H, d, J=8.0 Hz), 7.44 (2H, d, J=8.0 Hz), 7.61 (1H, d, J=8.0 Hz), 7.85 (1H, s), 8.14 (1H, d, J=8.0 Hz), 8.89 (1H, s). |
| 61% |
With potassium carbonate; In acetonitrile; at 100℃; for 5h; |
[Example 44] Preparation of 1-(4-chlorobenzyl)-7-(phenylamino)-3-(ethoxycarbonyl)quinoline-4(1H)-one (I-148) To a mixture of 7-bromo-3-(ethoxycarbonyl)quinoline-4(1H)-one (250 mg, 0.844 mmol) and acetonitrile (5 mL) were added potassium carbonate (175 mg, 1.27 mmol) and 4-chlorobenzylbromide (208 mg, 1.01 mmol), and the resulting mixture was stirred at 100C for 5 hours. The insoluble were removed by filtration and washed by ethyl acetate. The mother liquor was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (ethyl acetate/hexane) to give 7-bromo-1-(4-chlorobenzyl)-3-(ethoxycarbonyl)quinoline-4(1H)-one (216 mg, Yield: 61%) as colorless solid. 1H-NMR (delta ppm TMS/DMSO-d6): 1.29 (3H, t, J=6.0 Hz), 4.24 (2H, q, J=6.0 Hz), 5.69 (2H, s), 7.28 (2H, d, J=8.0 Hz), 7.44 (2H, d, J=8.0 Hz), 7.61 (1H, d, J=8.0 Hz), 7.85 (1H, s), 8.14 (1H, d, J=8.0 Hz), 8.89 (1H, s). |
| 61% |
With potassium carbonate; In acetonitrile; at 100℃; for 5h; |
EXAMPLE 44 Preparation of 1-(4-chlorobenzyl)-7-(phenylamino)-3-(ethoxycarbonyl)quinoline-4(1H)-one (I-148) To a mixture of 7-promo-3-(ethoxycarbonyl)quinoline-4(1H)-one (250 mg, 0.844 mmol) and acetonitrile (5 mL) were added potassium carbonate (175 mg, 1.27 mmol) and 4-chlorobenzylbromide (208 mg, 1.01 mmol), and the resulting mixture was stirred at 100 C. for 5 hours. The insoluble were removed by filtration and washed by ethyl acetate. The mother liquor was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (ethyl acetate/hexane) to give 7-bromo-1-(4-chlorobenzyl)-3-(ethoxycarbonyl)quinoline-4(1H)-one (216 mg, Yield: 61%) as colorless solid. 1H-NMR (delta ppm TMS/DMSO-d6): 1.29 (3H, t, J=6.0 Hz), 4.24 (2H, q, J=6.0 Hz), 5.69 (2H, s), 7.28 (2H, d, J=8.0 Hz), 7.44 (2H, d, J=8.0 Hz), 7.61 (1H, d, J=8.0 Hz), 7.85 (1H, s), 8.14 (1H, d, J=8.0 Hz), 8.89 (1H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +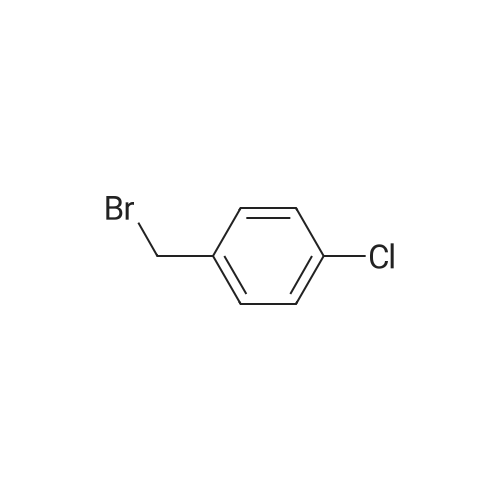

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping