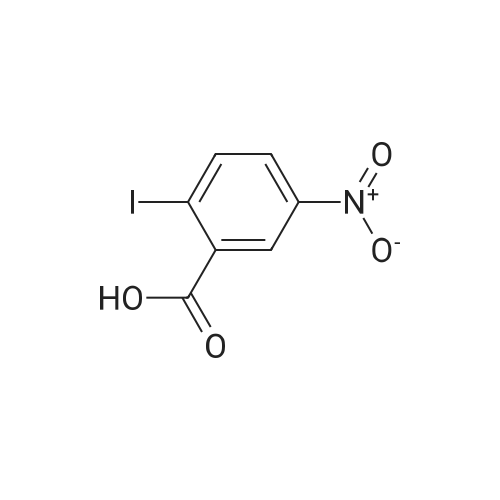
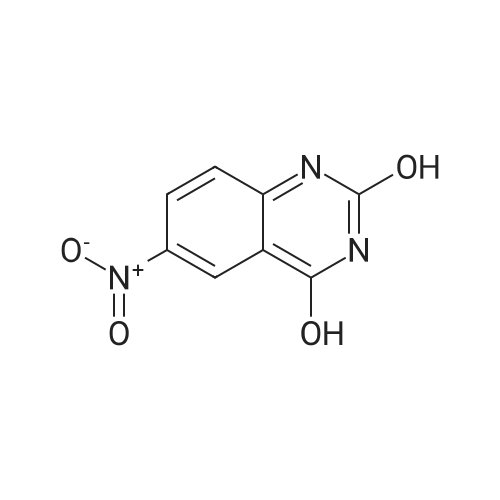
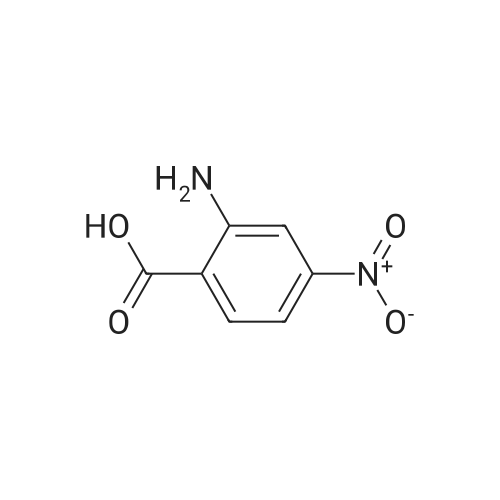
| 95% |
at 160℃; for 4h;Microwave irradiation; |
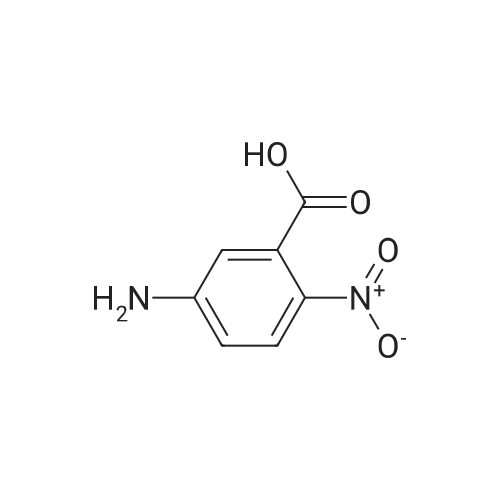
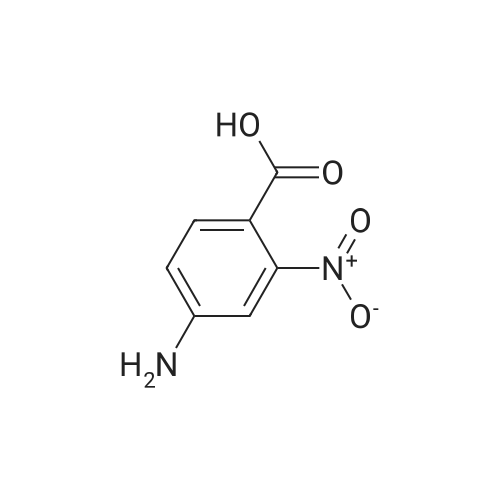
A mixture of 2-Amino-5-nitrobenzoic acid (3200 mg, 17.04 mmol) and urea (10338.4 mg, 170.43 mmol) was stirred for 4 hours at 1 60 C in a CEM microwave apparatus. The reaction crude was triturated in water (20 ml) and filtered. (0393) Resulting solid was separated by filtration and aqueous filtrate extracted twice with ethyl acetate (2 x 25 ml). Combined organic layers were dried over Na2S04, filtered, concentrated to dryness at low pressure and resulting solid mixed with the previous one obtained by filtration. Final trituration in methanol (5 ml) and filtration of combined solids afforded pure title compound (3353.7 mg, yield 95 %). Rt = 1 .25 min; MS (ESI) m/z: 206.0 [M-H]", [M-H]" calculated: 206.0. 1H NMR (400 MHz, DMSO-c/e) delta 1 1 .72 (s, 2H), 8.59 (d, J = 2.7 Hz, 1 H), 8.45 (dd, J = 9.0, 2.7 Hz, 1 H), 7.32 (d, J = 9.0 Hz, 1 H). |
| 94% |
In water; at 150 - 200℃;Sonographic reaction; |
Step A: Preparation of 6-nitro-1H-quinazoline-2,4-dione. Urea (9.89 g, 0.165 mol) and 5-nitroanthranilic acid (6.00 g, 32.9 mmol) were heated to 200 C. with vigorous stirring for 1 h. The melt was allowed to cool to 150 C., and water (150 mL) was slowly added. The resulting slurry was sonicated for 1 h and stirred vigorously for an additional 2 h. It was then cooled to 0 C., and the precipitate was collected and rinsed with water to yield the titled compound (6.43 g, 94% yield). This material was dried in a vacuum oven and used without further purification. This compound did not yield MS data. |
| 80% |
at 150℃; for 10h; |
The mixture of 2-amino-5-nitrobenzoic acid (10.0 g, 0.055 mol) and urea (32.2 g, 0.54 mol) was stirred at 150 C for 10 h. The reaction mixture was cooled to 100 C and then water (50 mL) was added to quench the reaction. The crude product was obtained by filtration, and then washed with water (50 mL × 3). After dried under vacuum condition, compound 2a was obtained as yellow solid (9.1 g, 80%); mp >300 C; 1H NMR (DMSO-d6) delta 7.24 (d, J = 9.0 Hz, 1H, ArH), 8.36 (dd, J1 = 9.0 Hz, J2 = 2.7 Hz, 1H, ArH), 8.55 (d, J = 2.7 Hz, 1H, ArH). |
| 79.4% |
at 200℃; for 0.166667h;Microwave irradiation; |
The compound 2-amino-5-nitrobenzoic acid (1.5 g, 8.24 mmol) was weighed in a microwave tube and urea (2.23 g, 37.1 mmol) was added to perform microwave reaction (power: 200 W; temperature: 200 C.). The reaction 10min, water 30ml, filtered, the filter cake washed with water 30ml × 3, dried to give a yellow solid 9.1g, yield: 79.4%. |
| 75% |
at 160℃; for 12h; |
In a dry 100 mL round bottom flask, 9.11 g of 2-amino-5-nitrobenzoic acid and 21.62 g of urea were added, reacted at 160 C for 12 h, cooled to 100 C, and added with 20 mL of water and stirred for 1 h. Cool to room temperature, suction and wash with water to give a crude material. The crude product was stirred in 100 mL of 1 mol/L sodium hydroxide solution for 1 h, filtered and dried to give 7.8 g of 6-nitro-2,4-(1Eta,3Eta)-quinazolinedione, yield 75%. |
| 72.8% |
|
Example 896-Nitroquinazoline-2,4(lH,3H)-dione A mixture of 2-amino-5-nitrobenzoic acid (0.588 g, 3.23 mmol) and urea (1.164 g, 19.38 mmol) was heated at 200C under N2 for 1 h. The mixture was cooled to room temperature and 4 M NaOH was added until pH = 14. It was acidified to pH = 5.0 via addition of AcOH. The mixture was filtered and the yellow solid was dried to give the title compound (0.49 g, 72.8%) as a yellow solid. MS: m/z 208.1 [M+l] +. |
| 72.8% |
at 200℃; for 1h;Inert atmosphere; |
A mixture of 2-amino-5-nitrobenzoic acid (0.588 g, 3.23 mmol) and urea (1.164 g, 19.38 mmol) was heated at 200 C. under N2 for 1 h. The mixture was cooled to room temperature and 4 M NaOH was added until pH=14. It was acidified to pH=5.0 via addition of AcOH. The mixture was filtered and the yellow solid was dried to give the title compound (0.49 g, 72.8%) as a yellow solid. MS: m/z 208.1 [M+1]+. |
| 53% |
at 160℃; for 48h; |
A mixture of 14 (10.0 g, 54.91 mmol) and urea (98.93 g, 1647.2mmol) was heated to 160 C for 48 h. After complete consumption of starting material, the reaction mixture was cooled to 100 C and water (10 mL) was added to the reaction mixture. The reaction mixture was filtered and washed with water; solid residue was dissolvedin 0.5 N NaOH solution. Then the reaction mixture was heated at 100 C for 40 min. The reaction mixture was cooled to0 C and 1 N HCl (aq.) was added to adjust pH = 5. Light yellow precipitatewas filtered, washed with water and dried under vacuumto give compound 15a (6.0 g, 53%) as a light yellow solid. MS(ESI) m/z: 206.1 (MH). 1H NMR (400 MHz, DMSO-d6): d 7.31(d, 1H, J = 9.04 Hz), 8.44 (dd, 1H, J = 2.60 Hz, 8.96 Hz), 8.57 (d, 1H,J = 2.64 Hz), 11.66 (br s, 2H). |
|
at 160℃;Inert atmosphere; Sealed tube; |
A mixture of 2-amino-5-nitro-benzoic acid 7 (1.5 g, 1.0 equiv) and urea (10 equiv) was heated at 160 C in a sealed tube. The reaction was stirred until consumption of starting material (TLC monitoring). The mixture was cooled down to rt, quenched with H2O (20 ml) and the obtained precipitate was collected by filtration, washed with H2O (3 * 20 ml) and dried under vacuum to give titled compound 8 as a pale brown solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping