| 93% |
In dimethyl sulfoxide; |
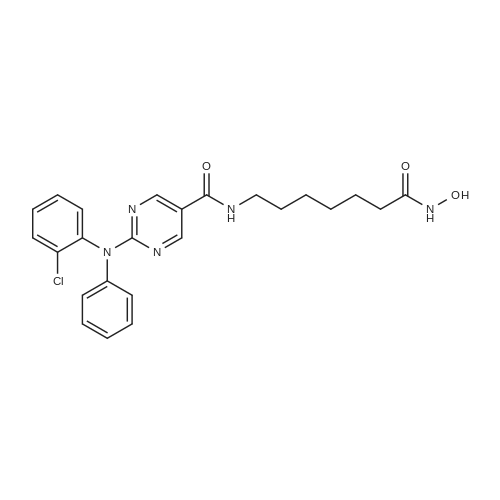
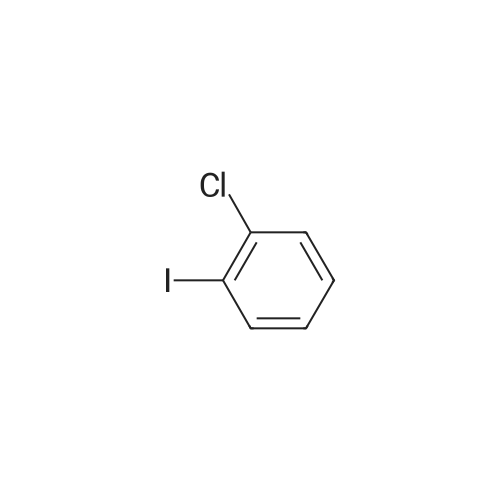
Example 2 Synthesis of 2-((2-chlorophenyl)(phenyl)amino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide (Compound B) Synthesis of Intermediate 2: See synthesis of intermediate 2 in Example 1. Synthesis of Intermediate 3: A mixture of compound 2 (69.2 g, 1 equiv.), 1-chloro-2-iodobenzene (135.7 g, 2 equiv.), Li2CO3 (42.04 g, 2 equiv.), K2CO3 (39.32 g, 1 equiv.), Cu (1 equiv. 45 mum) in DMSO (690 ml) was degassed and purged with nitrogen. The resulting mixture was stirred at 140° C. Work-up of the reaction gave compound 3 at 93percent yield. Synthesis of Intermediate 4: See synthesis of intermediate 4 in Example 1. Synthesis of Intermediate 6: See synthesis of intermediate 6 in Example 1. |
|
With nitrogen; potassium carbonate; aniline; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; |
Example 1 Synthesis of 2-((2-chlorophenyl)(phenyl)amino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide (Compound A) Reaction Scheme: Experimental Procedure Synthesis of Intermediate 2: A mixture of aniline (3.7 g, 40 mmol), compound 1 (7.5 g, 40 mmol), and K2CO3 (11 g, 80 mmol) in DMF (100 ml) was degassed and stirred at 120° C. under N2 overnight. The reaction mixture was cooled to r.t. and diluted with EtOAc (200 ml), then washed with saturated brine (200 ml*3). The organic layers were separated and dried over Na2SO4, evaporated to dryness and purified by silica gel chromatography (petroleum ethers/EtOAc=10/1) to give the desired product as a white solid (6.2 g, 64percent). Synthesis of Intermediate 3: A mixture of compound 2 (69.2 g, 1 equiv.), 1-chloro-2-iodobenzene (135.7 g, 2 equiv.), Li2CO3 (42.04 g, 2 equiv.), K2CO3 (39.32 g, 1 equiv.), Cu (1 equiv. 45 mum) in DMSO (690 ml) was degassed and purged with nitrogen. The resulting mixture was stirred at 140° C. Work-up of the reaction gave compound 3 at 93percent yield. Synthesis of Intermediate 4: 2N NaOH (200 ml) was added to a solution of compound 3 (3.0 g, 9.4 mmol) in EtOH (200 ml). The mixture was stirred at 60° C. for 30 min After evaporation of the solvent, the solution was neutralized with 2N HCl to give a white precipitate. The suspension was extracted with EtOAc (2*200 ml), and the organic layers were separated, washed with water (2*100 ml), brine (2*100 ml), and dried over Na2SO4. Removal of the solvent gave a brown solid (2.5 g, 92percent). Synthesis of Intermediate 6: A mixture of compound 4 (2.5 g, 8.58 mmol), compound 5 (2.52 g, 12.87 mmol), HATU (3.91 g, 10.30 mmol), and DIPEA (4.43 g, 34.32 mmol) was stirred at r.t. overnight. After the reaction mixture was filtered, the filtrate was evaporated to dryness and the residue was purified by silica gel chromatography (petroleum ethers/EtOAc=2/1) to give a brown solid (2 g, 54percent). Synthesis of 2-((2-chlorophenyl)(phenyl)amino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide (Compound A): |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping