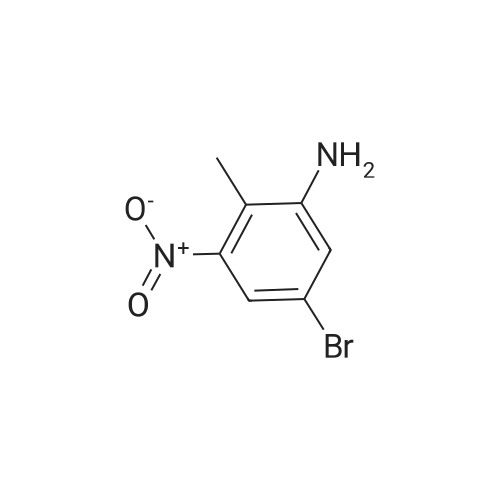
| 43% |
In tetrahydrofuran; at -40℃; for 0.666667h;Inert atmosphere; |
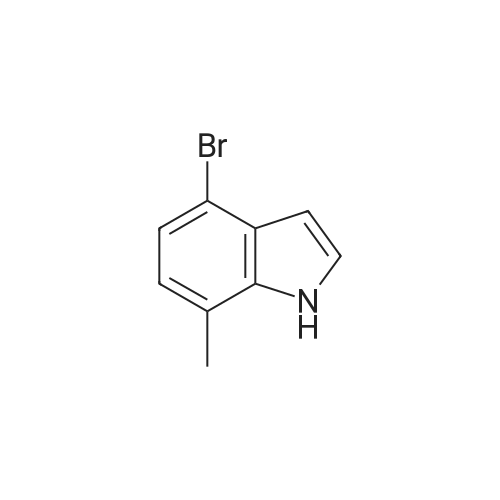
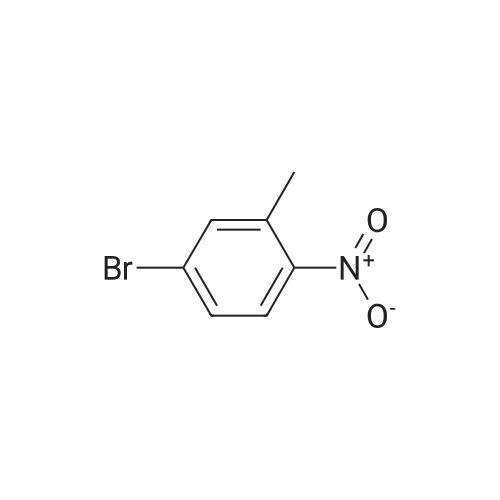
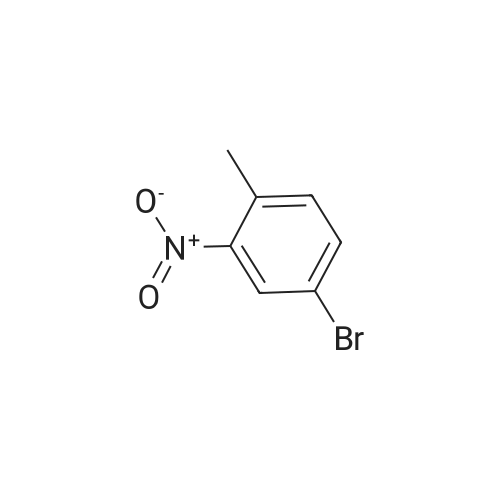
A solution of 4-bromo-1 -methyl-2-nitrobenzene (1 1 .57 mmol, 2.5 g) in THF (1 16 mL) under nitrogen atmosphere was cooled to -40 5C and vinylmagnesium bromide (46.28 mmol, 46 mL) was added. The reaction mixture was stirred for 40 min and quenched with saturated aqueous NH4CI. The aqueous layer was extracted with ethyl acetate twice and the combined organic layers were dry with Na2S04, filtered and concentrated. The crude product thus obtained was dissolved in THF (35 mL) and cooled to 0 5C. 0.5 N HCI (4.4 mL) was added and the reaction mixture stirred at 05C for 1 h, when it was quenched with NaHC03 (44 mL). The aqueous layer was extracted with ethyl acetate twice and the combined organic extracts were dried with Na2S04, filtered and concentrated. Purification of the crude material by flash chromatography on silica gel using an elution of 1 1 % ethyl acetate in hexanes afforded the pure title compound (1 .05 g. Yield: 43%) 1 H NMR (400 MHz, CDCI3) δ 8.21 (1 H, brs), 7.28-7.26 (1 H, m), 7.21 (1 H, d, J = 7.2 Hz), 6.87 (1 H, d, J = 6.8 Hz), 6.63-6.61 (1 H, m), 2.47 (3H, s). LC-MS: tR = 3.58 [M+H]+=not ion (method 3) |
| 43% |
|
A solution of 4-bromo-1-methyl-2-nitrobenzene (11.57 mmol, 2.5 g) in THF (116 mL) under nitrogen atmosphere was cooled to -40 C and vinyl magnesium bromide (46.28 mmol, 46 mL) was added. The reaction mixture was stirred for 40 min and quenched with saturated aqueous NH4Cl. The aqueous layer was extracted with ethyl acetate twice and the combined organic layers were dried with Na2SO4, filtered and concentrated. The crude product thus obtained was dissolved in THF (35 mL) and cooled to 0 C. 0.5 N HCl (4.4 mL) was added and the reaction mixture was stirred at 0 C for 1 h, then it was quenched with NaHCO3 (44 mL). The aqueous layer was extracted with ethyl acetate twice and the combined organic extracts were dried with Na2SO4, filtered and concentrated. Purification of the crude material by flash chromatography over silica gel using an elution of 11% ethyl acetate in hexanes afforded 1.05 g (Yield: 43%) of the pure title compound 25. 1H NMR (400 MHz, CDCl3): δ 8.21 (1H, brs), 7.28-7.26 (1H, m), 7.21 (1H, d, J = 7.2 Hz), 6.87 (1H, d, J = 6.8 Hz), 6.63-6.61 (1H, m), 2.47 (3H, s). |
|
|
Example 6: 2-(7-MethvI-lH-indoI-4-vIV6-(4-methyl-piperazin-l-ylmethyI)-4- morpholin-4-yl-thienof3,2-dlpyrimidine; To a solution of 4-bromo-2-nitrotoluene (3.0 g, 13.9 mmol) in THF (40 mL) at-40 C was added vinyl magnesium bromide (48.6 mmol, 48.6 mL; 1.0 M in THF) dropwise over 15 minutes and the mixture stirred at -40 0C for 1.5 hours. The reaction was quenched by the addition of saturated aqueous ammonium chloride and extracted with ethyl acetate. The combined organic layers were washed with brine, 20 separated and dried (MgSO4). The residue was evaporated and purified by column chromatography to yield 4-bromo-7-methylindole (1.03 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping