| 76.1% |
|
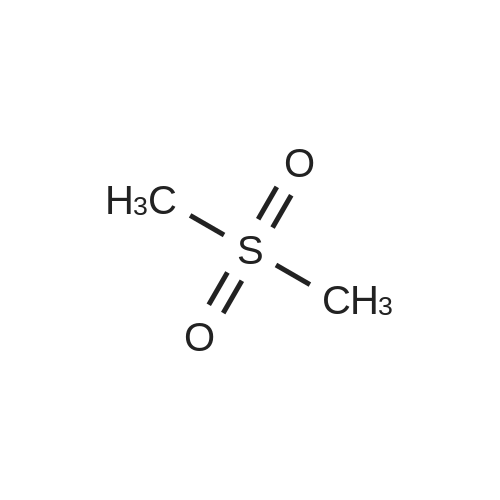
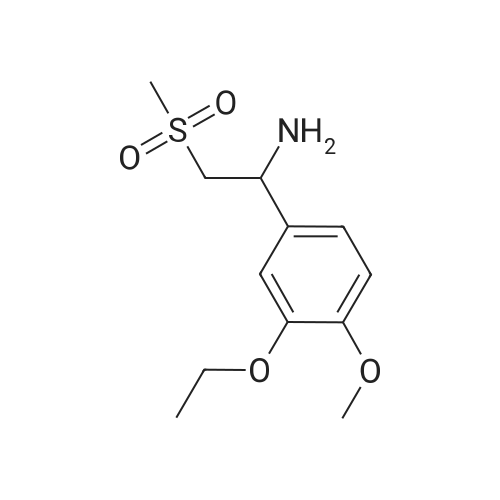
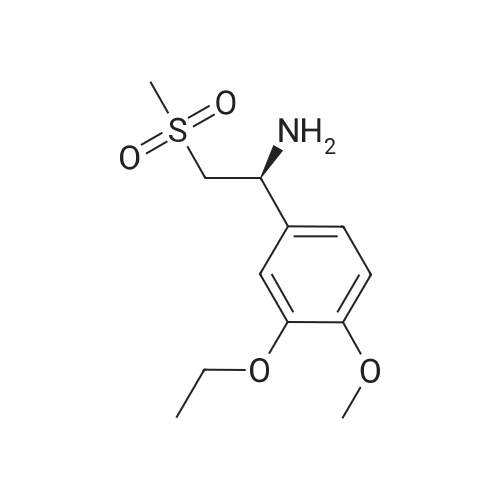
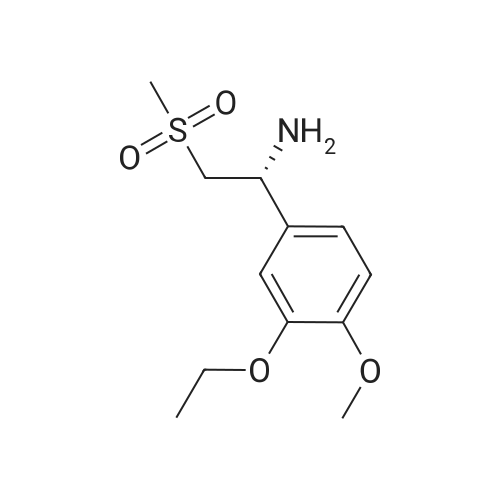
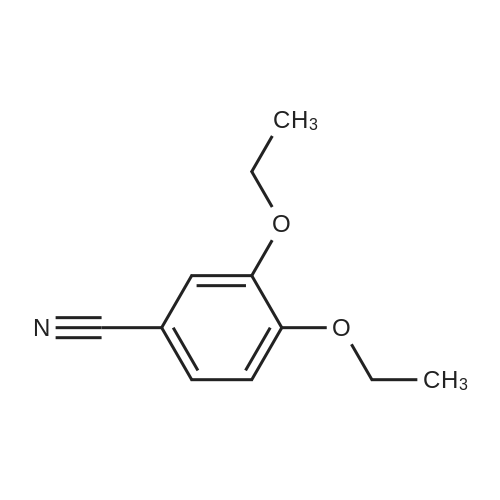
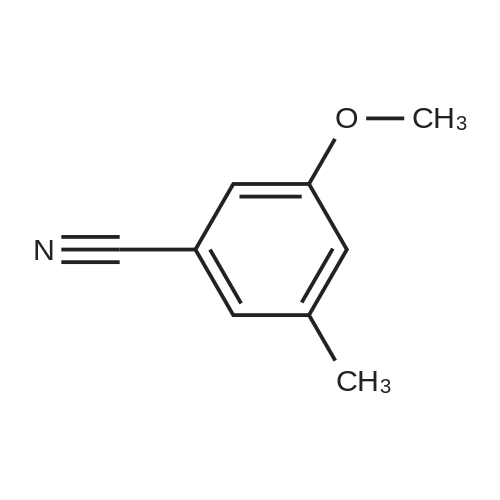
Dimethylsulfone (191. Ig, 2.03 moles, from Aldrich Chemicals, Milwaukee, WI) and tetrahydrofuran (1.65 L, from Aldrich Chemicals, Milwaukee, WI) were charged to a 12 L three-necked flask at room temperature. The mixture was cooled to 0-5C. n-BuLi (750 ml of 2.5M solution in hexanes, from Aldrich Chemicals, Milwaukee, WI) was added to the flask at a rate such that the reaction mixture was maintained at 0-5C. A line rinse with 150 ml tetrahydrofuran followed. The mixture was stirred at 0-5C for 60-70 minutes. 3-ethoxy-4- methoxybenzonitrile (300.0 g, 1.69 moles, in 750 ml tetrahydrofuran) was then charged to the flask at a rate such that the reaction mixture was maintained at 0-5 C . A line rinse with 300 ml tetrahydrofuran followed. The mixture was stirred at 0-5C for another 10-15 minutes. After warming to room temperature, the reaction mixture was stirred at room temperature for 1.5-2 hours, while purged with nitrogen. NaBH4 (83.1 g, 2.20 moles, from Aldrich Chemicals, Milwaukee, WI) and 150 ml of tetrahydrofuran were then charged to the reaction mixture. The reaction mixture was stirred at 0-50C for 15-30 minutes. HOAc (450 ml, 7.83 moles, from Fisher Scientific, Pittsburgh, PA) was charged to the flask at a rate such that the reaction mixture was maintained at 0-50C. The mixture was stirred at 0-50C for an additional 2-3 hours. The mixture was then charged with 2.25 L of NaOH (2.5N, pH 12 to 13, from Fisher Scientific, Pittsburgh, PA), and stirred at 0-50C for another 15-30 minutes. After warming to room temperature, the reaction mixture was heated to reflux at about 600C. After reflux for 12-14 hours, the mixture was cooled to 35-40C, and 3.0 L of water was added. The mixture was further cooled to 0-5C over a period of 1.5-2 hours. The mixture was filtered under vacuum, and the filtered solid was washed with 2 L of deionized water. The solid was dried in a tray at 50-550C under vacuum. The yield of 2-(3-ethoxy-4-methoxyphenyl)-l-(methanesulfonyl)-eth- 2-ylamine was found to be 352 g (76.1%) based on a 300 g input of 3-ethoxy-4- methoxybenzonitrile (HPLC indicated 99.74% purity by peak area). |
| 65% |
|
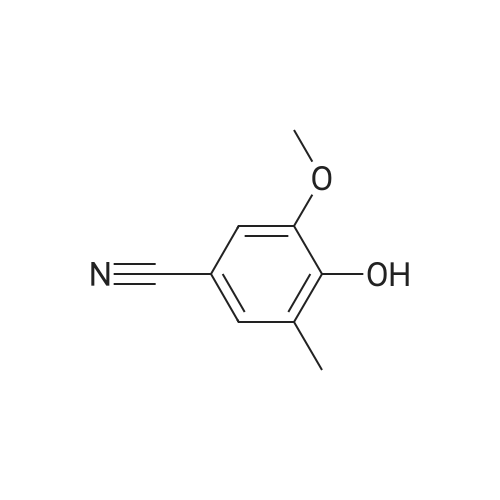
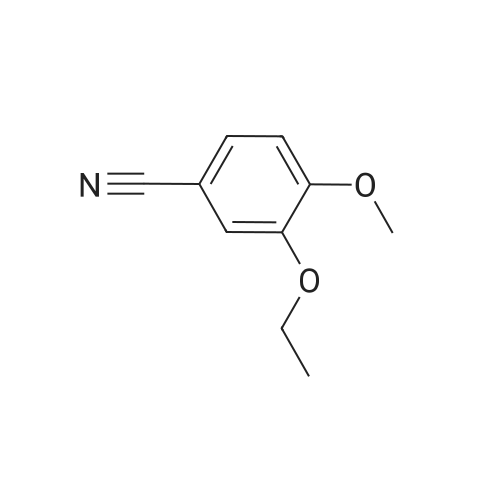
In a flask, 5 litres of tetrahydrofuran was charged followed by 1.06 kg of dimethylsulfone. This reaction mass was cooled for 25 to 30 minutes at 0C. Aftercooling, 1M potassium-hexamethyldisilazane was added followed by 10 litres of tetrahydrofuran. The reaction mass was stirred for an hour at 0 to 10 C. After stirring, 1 kg of 3-ethoxy-4-methoxybenzonitrile was dissolved in 2 litres tetrahydrofuran and was added to the above reaction mass. The reaction mass was stirred for 30 minutes and cooled. After cooling, 0.433 kg of sodium borohydride was added followed bytetrahydrofuran and 5 litres of acetic acid and the total reaction mass was stirred for 3- 4 hours at 0 to 10 C. After completion of reaction, sodium hydroxide solution was added to it and stirred for 45 minutes. The reaction mass was warmed and further heated for 3 to 4 hours at 60 to 65C. After completion of reaction, the reaction solution was allowed to cool to room temperature for half an hour. The layers were separated. The combinedorganic layer was treated with aq. HC1 and water was added to the concentrated mass. The aqueous layer was treated with ethyl acetate. Finally sodium hydroxide solution was added to the aqueous layer and solid was precipitated. The solid was filtered, washed with water and dried at 50C and further 1.0 kg (65%) of material was unloaded. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping