|
With thionyl chloride; In methanol; dichloromethane; water; |
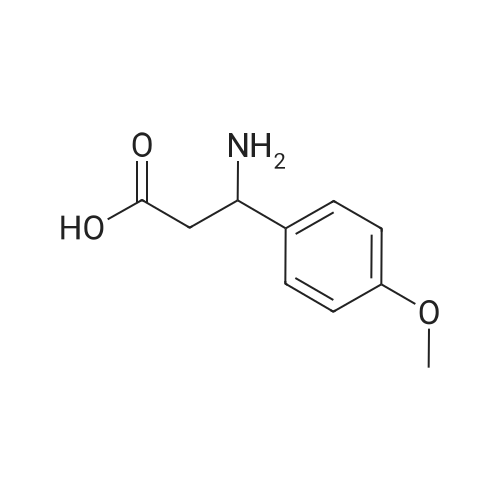
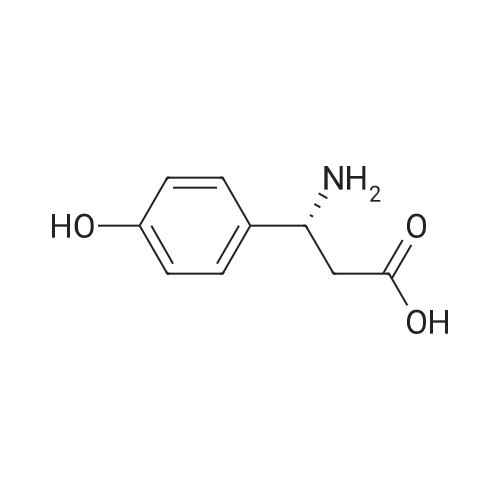
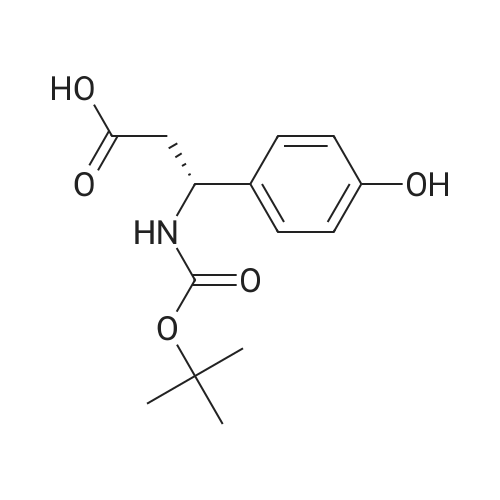
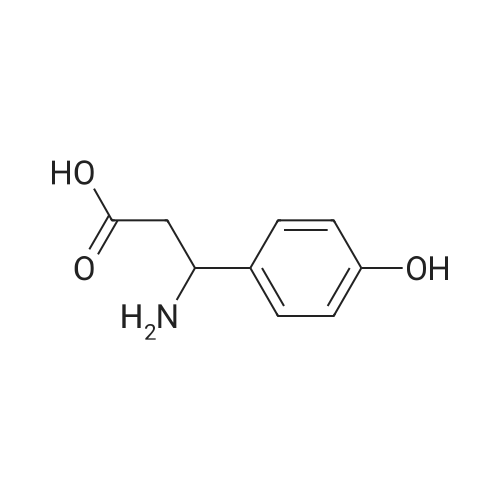
(i) Thionyl chloride (4.3 ml) was added to methanol (50 ml) cooled in an ice-salt bath. 3-Amino-3-(4-hydroxyphenyl)propionic acid (9.7 g) was added and the mixture allowed to reach ambient temperature and then refluxed for 2 hours. The solvent was removed by evaporation in vacuo to give a gummy solid (12.8 g) which was used without further purification. A solution of di-t-butyldicarbonate (5.8 g) in dichloromethane (50 ml) was added to a stirred mixture of the gummy solid (5.75 g) and potassium hydrogen carbonate (6.2 g) in water (20 ml). The mixture was stirred at ambient temperature for 4 hours. The organic layer was separated and washed with water (10 ml), 1M hydrochloric acid solution (10 ml), saturated sodium hydrogen carbonate solution (10 ml), water (10 ml) and dried (MgSO4). The solvent was evaporated to give methyl 3-(t-butyloxycarbonylamino)-3-(4-hydroxyphenyl)propionate as a solid; m.p. 119-120 C.; NMR(d6 DMSO): 1.34(s,9H), 2.54-2.78(m,2H), 3.53(s,3H), 4.7-4.9(q,1H), 6.67(d,2H), 7.08(d,2H), 7.28(brd,1H), 9.24(brs,1H). |
|
With thionyl chloride; In methanol; dichloromethane; water; |
(i) Thionyl chloride (4.3 ml) was added to methanol (50 ml) cooled in an ice-salt bath. 3-Amino-3-(4-hydroxyphenyl)propionic acid (9.7 g) was added and the mixture allowed to reach ambient temperature and then refluxed for 2 hours. The solvent was removed by evaporation in vacuo to give a gummy solid (12.8 g) which was used without further purification. A solution of di-t-butyldicarbonate (5.8 g) in dichloromethane (50 ml) was added to a stirred mixture of the gummy solid (5.75 g) and potassium hydrogen carbonate (6.2 g) in water (20 ml). The mixture was stirred at ambient temperature for 4 hours. The organic layer was separated and washed with water (10 ml), 1M hydrochloric acid solution (10 ml), saturated sodium hydrogen carbonate solution (10 ml), water (10 ml) and dried (MgSO4). The solvent was evaporated to give methyl 3-(t-butyloxycarbonylamino)-3-(4-hydroxyphenyl)propionate as a solid; m.p. 119-120 C.; NMR(d6 DMSO): 1.34(s,9H), 2.54-2.78(m,2H), 3.53(s,3H), 4.7-4.9(q,1H), 6.67(d,2H), 7.08(d,2H), 7.28(brd, 1H), 9.24(brs,1H). |
|
With thionyl chloride; In methanol; dichloromethane; water; |
(i) Thionyl chloride (4.3 ml) was added to methanol (50 ml) cooled in an ice-salt bath. 3-Amino-3-(4-hydroxyphenyl)propionic acid (9.7 g) was added and the mixture allowed to reach ambient temperature and then refluxed for 2 hours. The solvent was removed by evaporation in vacuo to give a gummy solid (12.8 g) which was used without further purification. A solution of di-t-butyldicarbonate (5.8 g) in dichloromethane (50 ml) was added to a stirred mixture of the gummy solid (5.75 g) and potassium hydrogen carbonate (6.2 g) in water (20 ml). The mixture was stirred at ambient temperature for 4 hours. The organic layer was separated and washed with water (10 ml), 1M hydrochloric acid solution (10 ml), saturated sodium hydrogen carbonate solution (10 ml), water (10 ml) and dried (MgSO4). The solvent was evaporated to give methyl 3-(t-butyloxycarbonylamino)-3-(4-hydroxyphenyl)propionate as a solid; m.p. 119-120 C.; NMR(d6 DMSO): 1.34(s,9H), 2.54-2.78(m,2H), 3.53(s,3H), 4.7-4.9(q,1H), 6.67(d,2H), 7.08(d,2H), 7.28(brd,1H), 9.24(brs,1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping