| 91% |
With pyridine In dichloromethane at 15 - 25℃; |
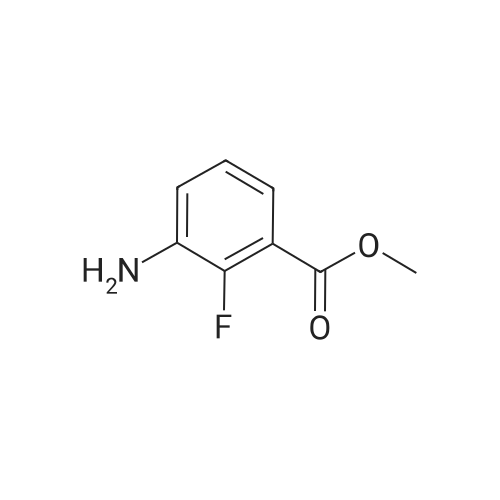
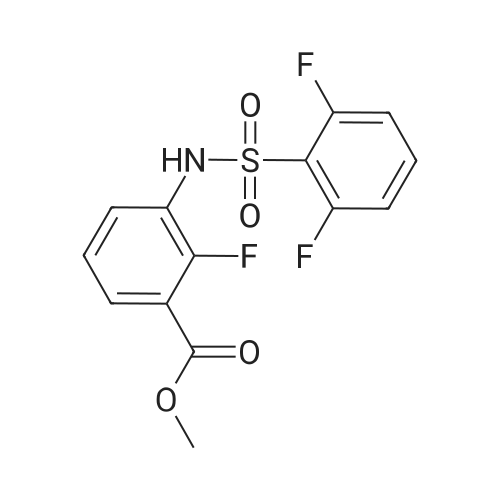
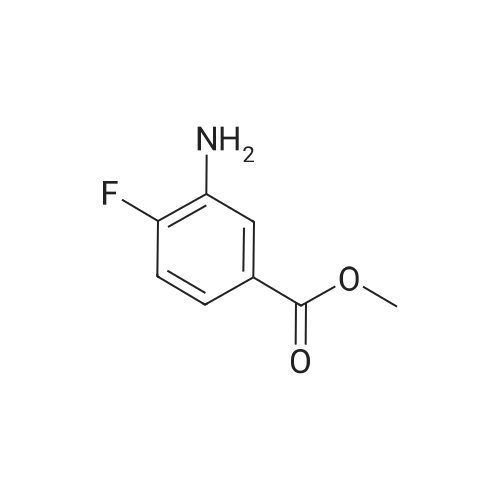
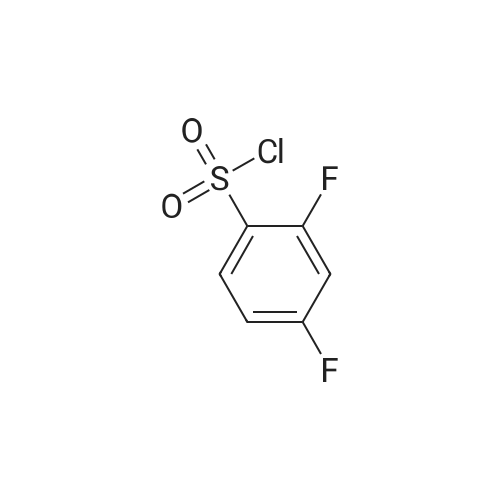
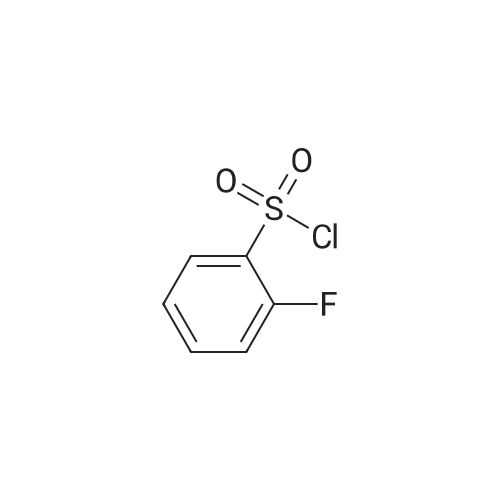
Step A: methyl 3-[(2,6-difluorophenyl)sulfonyl]amino}-2-fluorobenzoate Methyl 3-amino-2-fluorobenzoate (50 g, 1 eq) was charged to reactor followed by dichloromethane (250 mL, 5 vol). The contents were stirred and cooled to ~15°C and pyridine (26.2 mL, 1.1 eq) was added. After addition of the pyridine, the reactor contents were adjusted to ~15°C and the addition of 2,6-diflurorobenzenesulfonyl chloride (39.7 mL, 1.0 eq) was started via addition funnel. The temperature during addition was kept <25°C. After complete addition, the reactor contents were warmed to 20-25°C and held overnight. Ethyl acetate (150 mL) was added and dichloromethane was removed by distillation. Once distillation was complete, the reaction mixture was then diluted once more with ethyl acetate (5 vol) and concentrated. The reaction mixture was diluted with ethyl acetate (10 vol) and water (4 vol) and the contents heated to 50-55°C with stirring until all solids dissolve. The layers were settled and separated. The organic layer was diluted with water (4 vol) and the contents heated to 50-55° for 20-30 min. The layers were settled and then separated and the ethyl acetate layer was evaporated under reduced pressure to ~3 volumes. Ethyl Acetate (5 vol.) was added and again evaporated under reduced pressure to ~3 volumes. Cyclohexane (9 vol) was then added to the reactor and the contents were heated to reflux for 30 min then cooled to 0 °C. The solids were filtered and rinsed with cyclohexane (2 x 100 mL). The solids were air dried overnight to obtain methyl 3-[(2,6-difluorophenyl)sulfonyl]amino}-2-fluorobenzoate (94.1 g, 91percent). |
| 91% |
With pyridine In dichloromethane at 15 - 25℃; |
Step A: methyl 3-[(2,6-difluorophenyl)sulfonyl]amino}-2-fluorobenzoate: Methyl 3-amino-2-fluorobenzoate (50 g, 1 eq) was charged to reactor followed by dichloromethane (250 mL, 5 vol). The contents were stirred and cooled to -15 °C and pyridine (26.2 mL, 1.1 eq) was added. After addition of the pyridine, the reactor contents were adjusted to ~15°C and the addition of 2,6-diflurorobenzenesulfonyl chloride (39.7 mL, 1.0 eq) was started via addition funnel. The temperature during addition was kept <25 °C. After complete addition, the reactor contents were warmed to 20-25 °C and held overnight. Ethyl acetate (150 mL) was added and dichloromethane was removed by distillation. Once distillation was complete, the reaction mixture was then diluted once more with ethyl acetate (5 vol) and concentrated. The reaction mixture was diluted with ethyl acetate (10 vol) and water (4 vol) and the contents heated to 50-55 °C with stirring until all solids dissolve. The layers were settled and separated. The organic layer was diluted with water (4 vol) and the contents heated to 50-55 °C for 20-30 min. The layers were settled and then separated and the ethyl acetate layer was evaporated under reduced pressure to ~3 volumes. Ethyl Acetate (5 vol.) was added and again evaporated under reduced pressure to ~3 volumes. Cyclohexane (9 vol) was then added to the reactor and the contents were heated to reflux for 30 min then cooled to 0 °C. The solids were filtered and rinsed with cyclohexane (2 x 100 mL). The solids were air dried overnight to obtain methyl 3- [(2,6-difluorophenyl)sulfonyl]amino}-2-fluorobenzoate (94.1 g, 91percent). |
| 87% |
With pyridine In dichloromethane at 20℃; |
Step C: Methyl 3-[(2,6-difluorophenyl)sulfonyl]amino}-2-fluorobenzoate; In a 500 mL flask was placed methyl 3-amino-2-fluorobenzoate (5.5 g, 32.5 mmol) and DCM (100 mL), and pyridine (2.9 mL, 35.8 mmol) was added. 2,6-Difluorobenzenesulfonyl chloride (7.6 g, 35.8 mmol) in DCM (50 mL) was added dropwise via addition funnel and the reaction mixture was allowed to stir at rt overnight. The reaction mixture was stripped onto silica and column chromatographed on silica with 5percent to 100percent EtOAc:Hexane to give 9.75 g (87percent) of the title compound of Step C. 1H-NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 7.64-7.82 (m, 3H), 7.46-7.61 (m, 1H), 7.29 (t, J=8.8 Hz, 2H), and 3.81 (s, 3H). MS (ESI): 346 [M+H]+. |
| 87% |
With pyridine In dichloromethane at 20℃; Inert atmosphere |
In a 500 mL flask was placed methyl 3-amιno-2-fluorobenzoate (5 5 g, 32 5 mmol) and DCM (100 mL) and pyridine (2 9 mL, 35 8 mmol) was added 2,6-10 Difluorobenzenesulfonyl chloride (7 6 g, 35 8 mmol) in DCM (50 mL) was added dropwise via addition funnel and the reaction mixture was allowed to stir at rt overnight The reaction mixture was stripped onto silica and column chromatographed on silica with 5percent to 100percent EtOAc Hexane to give 9 75 g (87percent) of the title compound of Step C 1H-NMR (400 MHz, DMSO-ds) δ 10 98 (s, 1 H), 7 64 -15 7 82 (m, 3 H), 746 - 7 61 (m, 1 H), 7 29 (t, J = 8 8 Hz, 2 H), and 3 81 (s, 3 H) MS (ESI) 346 [M+H]+ |
| 82% |
With pyridine; dmap In dichloromethane at 20℃; for 12 h; Inert atmosphere |
thane (100 mL), pyridine (3.2 g, 0.040 mol) and DMAP (0.1 g), nitrogen , under ice-cooling was added with a dropping funnel, 2,6-difluorophenyl 1-sulfonyl chloride (7.2 g, 0.034 mol).After the addition was complete, slowly warm to room temperature and stirred for 12 hours.The organic phase was washed with water three times (20 mL × 3), dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, the residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 10: 1) was isolated as the product (9.5 g, yield 82percent) |
| 62% |
With pyridine In dichloromethane at 0 - 20℃; |
A solution of Methyl 3-amino-2-fluorobenzoate (2.0g, 11.82mmol) in DCM (40mL) was treated at 0°C with pyridine (1.91mL, 23.65mmol) and with 2,6-difluorobenzenesulfonyl chloride (2.40mL, 17.17mmol). The reaction mixture was stirred at room temperature overnight. The medium was poured onto water, and aqueous HC1 (1M) was added. The aqueous layer was extracted with DCM. Combined organics were washed with saturated aqueous sodium bicarbonate, dried over sodium sulphate, filtered and the filtrate was concentrated under reduced pressure. Purification by flash chromatography on silica (DCM/cHex 8/2 to 10/0) afforded the title compound (2.55g, 62percent). LC/MS (ES"): 344.2 (M-l). |
| 6.91 g |
at 15 - 20℃; |
To a cooled solution of 1,3-difluorobenzene (9.4 g, 82 mmol) in diethyl ether (120 mL) was added n-BuLi in hexane (32.3 mL, 81 mmol) dropwise at ?78° C. The reaction mixture was stirred at ?78° C. for 3 h. Sulfur dioxide (106 g, 1648 mmol) was flushed into the solution and stirred at ?60° C. for 20 mins, which produced a white solid. NCS (12.10 g, 91 mmol) was added and the reaction mixture was warmed to rt and for 1 h. The reaction mixture with white solid changed to pale brown solution. The reaction mixture was filtered and concentrated to give crude No.34 ? 2,6-difluorobenzenesulfonyl chloride, which was added dropwise into No.35 ? methyl 3-amino-2-fluorobenzoate (5.19 g, 30.7 mmol) in No.36 ? pyridine (50 mL) at 15° C. After the addition, the reaction mixture was stirred at room temperature overnight. No.38 ? Ethyl acetate (100 mL) was added to the reaction mixture and then followed by 40 mL of cold No.10 ? water. The mixture was heated to 55° C. with stirring until all red solids dissolved. The upper layer was organic layer which was isolated and washed again with water. The organic layer was dried and evaporated under reduced pressure. Petro ether was added to the crude product and heated to reflux and then cooled to 5° C. The solids were filtered and rinsed with petro ether to afford No.39 ? methyl 3-(2,6-difluorophenylsulfonamido)-2-fluorobenzoate (6.91 g, 10.35 mmol, 12.56percent yield) as orange solid. LCMS: [M+NH4]+=363.0. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +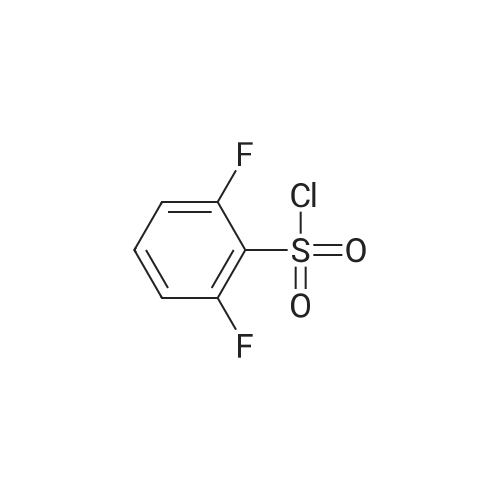

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping