|
In ethanol; dimethyl sulfoxide; at 65℃; for 0.5h; |
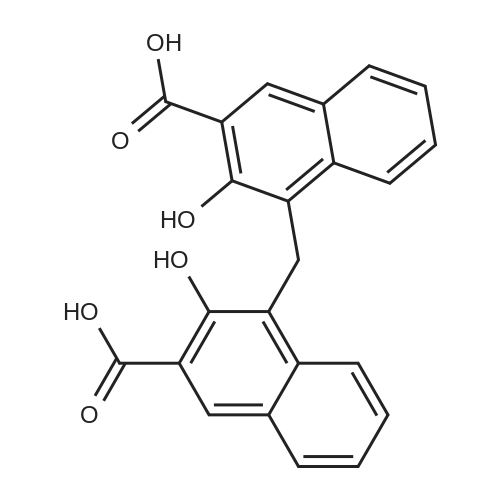
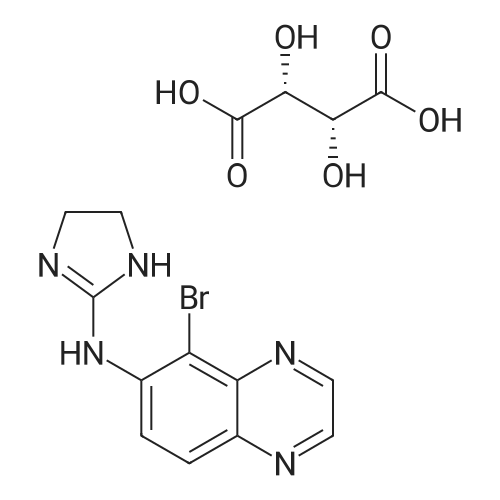
Brimonidine Pamoate Polymorph Form AIn a 5 L 3-neck round bottom flask equipped with overhead stirrer, heating mantle, condenser, temperature probe, and N2 inlet, 4.8 g of brimonidine (lot 1-080085) was dissolved in ethanol (2000 mL) at 65° C. <strong>[130-85-8]Pamoic acid</strong> (1.05 eq, 19.0 mL, 0.5M in DMSO (dimethyl sulfoxide)) was then added. The resulting solution was stirred for 30 minutes at 65° C. and then cooled at 20° C./hour to ambient temperature. At the onset of the cooling profile, precipitation of solids was observed. The mixture stirred overnight at ambient temperature and was then filtered. The resulting solids were dried under vacuum at ambient temperature for 4 days before being analyzed by XRPD to confirm the solid form, designated as Form A. FIG. 1 shows an XRPD spectrum of brimonidine pamoate polymorph Form A (lot SUC-I-130(1)).In one aspect, polymorph Form A is characterized by an XRPD spectrum comprising major peaks at 2psi angles of 13.5, 20.6, 21.1, and 24.4°+/-0.2°.In another aspect, polymorph Form A is characterized by an XRPD spectrum comprising peaks at 2psi angles of 7.6, 12.2, 12.7, 13.5, 20.6, 21.1, 24.4, 26.5, and 27.7°+/-0.2°.1H NMR analysis of this material showed approximately 3.7 wt percent residual ethanol and a 0.5:1 pamoate to brimonidine ratio confirming the formation of a hemi-pamoate salt of brimonidine. FIG. 2 shows an NMR spectrum for brimonidine pamoate polymorph Form A (lot SUC-I-130(1).Thermal analysis of Form A showed a single DSC endotherm at 221° C. (see FIG. 3) attributed to the melting of the salt and 3.5percent TGA weight loss through 190° C. (see FIG. 4) attributed to the removal of ethanol.FIG. 5 shows a Raman spectroscopy spectrum of Form A (lot SUC-I-130(1), to be compared to Raman spectra of other polymorphs.In one aspect, polymorph Form A is characterized by a Raman spectroscopy spectrum comprising major peaks at 1340.8, 1352.4, 1365.8, 1402.0, and 1460.3 cm-1.In another aspect, polymorph Form A is characterized by a Raman spectroscopy spectrum comprising peaks at 135.4, 169.3, 189.2, 233.0, 326.9, 547.9, 693.3, 719.4, 838.3, 938.3, 1031.1, 1197.6, 1252.4, 1270.2, 1340.8, 1352.4, 1365.8, 1402.0, 1460.3, 1549.0, 1556.0, and 1571.0 cm-1.Moisture sorption analysis of Form A showed this hemi-pamoate polymorph to be slightly hygroscopic, adsorbing 2.2 percent by weight ("wt percent") water at 60 percent relative humidity ("percent RH") and 2.5 wt percent water at 90percent RH. Upon desorption, no hysteresis or indication of hydrate formation was observed. XRPD analysis of the solids following moisture sorption analysis afforded a diffraction pattern which was consistent with the Form A starting material, indicating no polymorphic form conversion had occurred during the experiment. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science












 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping