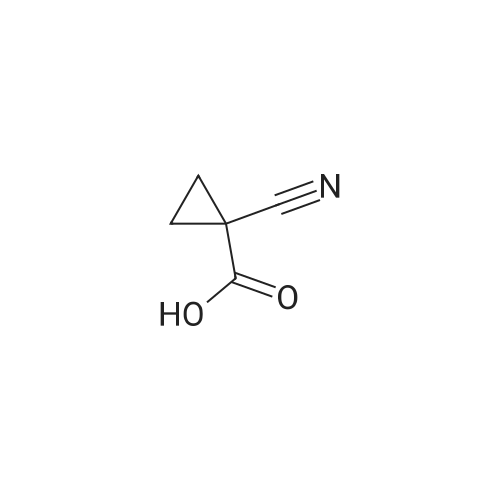
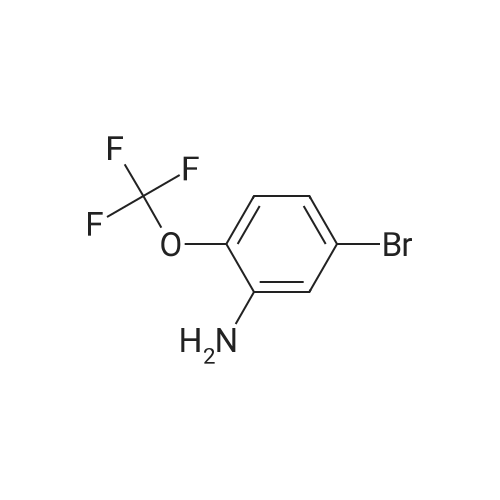
| 35% |
|
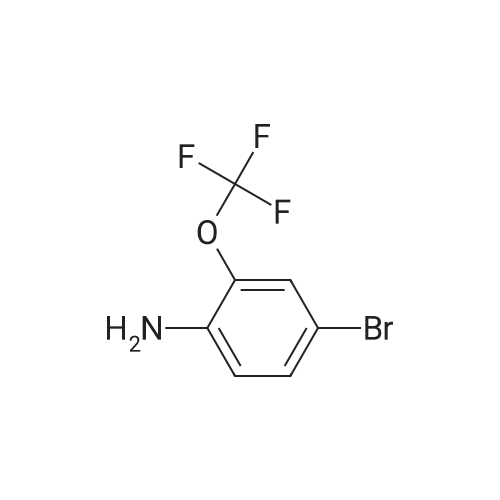
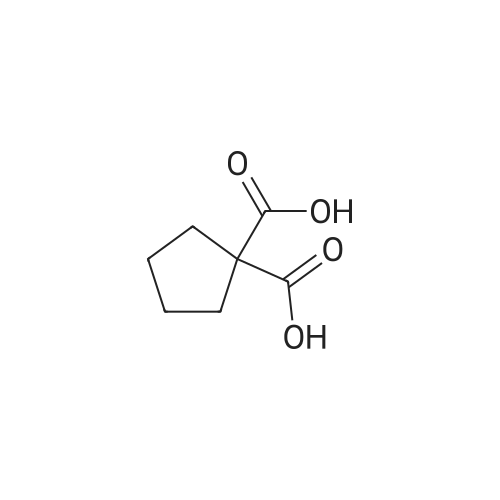
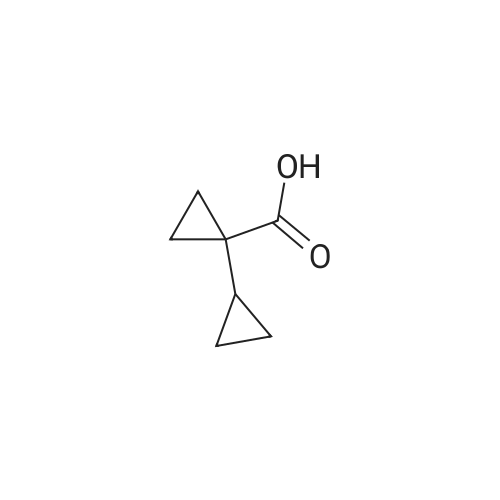
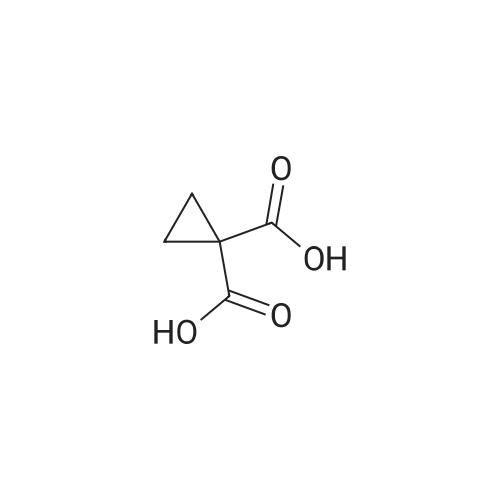
Under nitrogen conditions, 2.2mL dry triethylamine was added dropwise to 2g (15.4mmol) in methylene chloride solution of 1,1-cyclopropane dicarboxylic acid, the ice bath was stirred for 30min, then slowly dropping 1.2 mL SOCl2 methylene chloride solution. after the addition was complete stirring for 2h, the reaction mixture 71/92 3.5g (13.9mmol) 5- bromo-2-trifluoromethoxy aniline methylene chloride solution, and after the addition was complete stirring was continued for 2h. After completion of the reaction, the reaction mixture was 2mol / L NaOH solution was adjusted to pH 10, evaporated to dryness under reduced pressure, water was added q.s. ultrasonic, extracted once with ethyl acetate, the aqueous layer was retained, then 2mol / L HCl aqueous layer adjusted to pH 2, extracted three times with ethyl acetate, the organic solvent under reduced rotation, i.e., to give 1 - ([5-bromo-2- (trifluoromethoxy) phenyl] amino} carbonyl) cyclopropanecarboxylic acid acid, about 0.8g, 35% yield; |
| 35% |
|
Under nitrogen atmosphere, 2.2 mL of anhydrous triethylamine was added dropwise to a solution of 2 g (15.4 mmol) of 1,1-cyclopropanedicarboxylic acid in dichloromethane, and the mixture was stirred for 30 min in an ice bath and then 1.2 mL SOCl2 in dichloromethane,After the addition was complete, the mixture was stirred for 2h, and then 3.5g (13.9mmol) of <strong>[886762-08-9]5-bromo-2-trifluoromethoxyaniline</strong> in dichloromethane was added dropwise to the reaction mixture. After the addition was completed, stirring was continued for 2h. The reaction solution was then adjusted to pH 10 with 2 mol / L NaOH solution, evaporated to dryness under reduced pressure, sonicated with appropriate amount of water, extracted once with ethyl acetate, the aqueous layer was retained,The aqueous layer was further adjusted to pH 2 with 2 mol / L HCl and extracted three times with ethyl acetate.The organic solvent was removed under reduced pressure to give 1 - ([5-bromo-2- (trifluoromethoxy) phenyl] amino} carbonyl) cyclopropanecarboxylic acid, About 0.8g, yield 35%; |
|
|
General procedure: 1,1-Cyclopropanedicarboxylic acid (5) (12.0 g, 15.4 mmol) was dissolved in anhydrous THF (50 mL). Then triethanolamine (2.0 mL,13.9 mmol) was added to the mixture and the mixture was stirred on the ice-bath for 30 min. SOCl2 (1.2 mL, 16.66 mmol) was then added. Stirring was continued for 2h, a solution of 3,5-dimethylaniline 6 (3.6 g, 15.4 mmol) in anhydrous THF (10 mL) was added and continued stirring for 2h, after that, the ice bath was removed, and the mixture was stirred at room temperature overnight. After the completion of the reaction, the mixture was filtered and concentrated in vacuo. The residues was purified by silica gel flash chromatography (PE/AcOEt = 5:1) to yield 7 as white solid (0.8 g, 30.7%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping