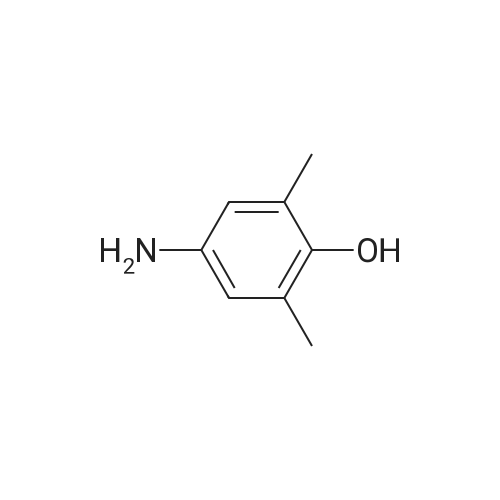
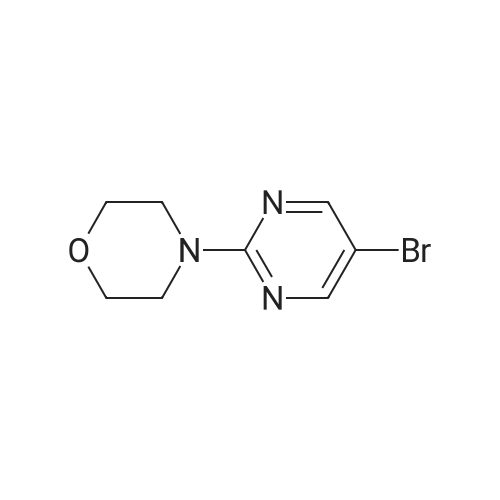
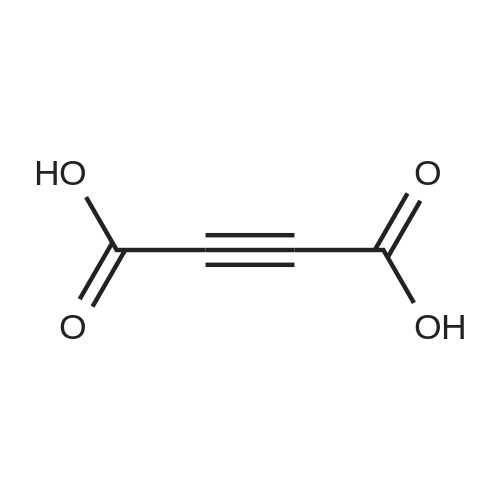
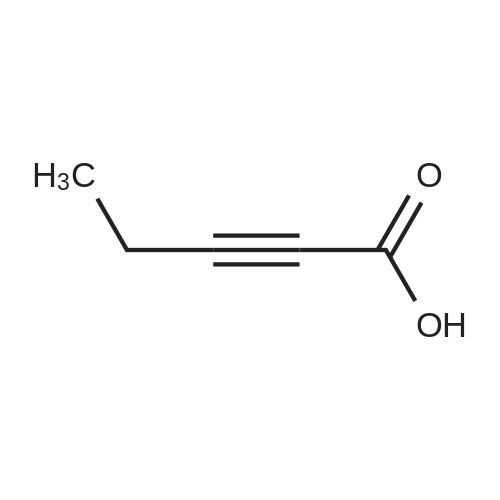
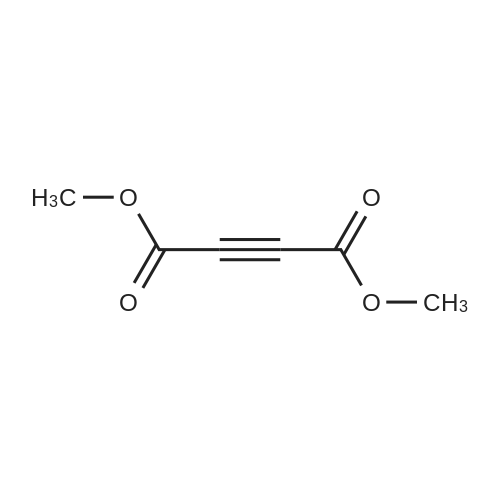
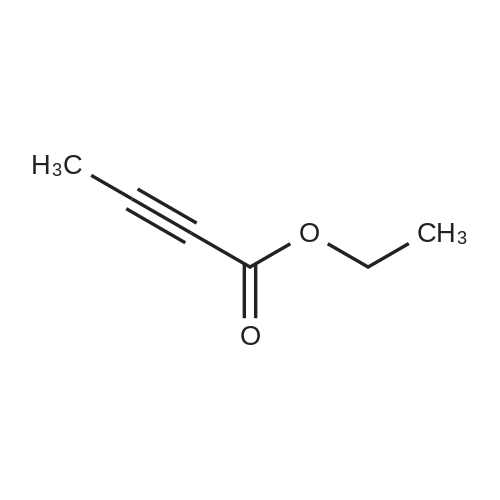
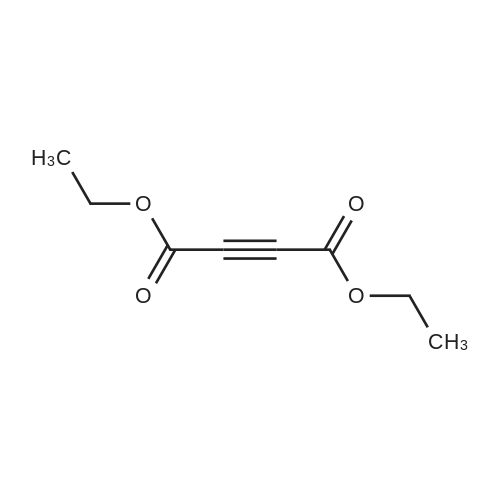
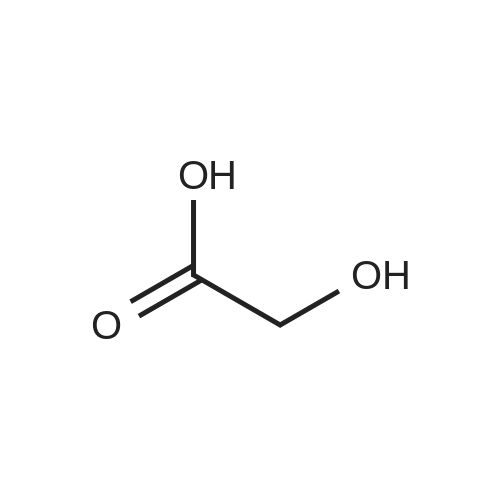
| 90% |
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In dichloromethane; at 20 - 30℃; for 3h; |
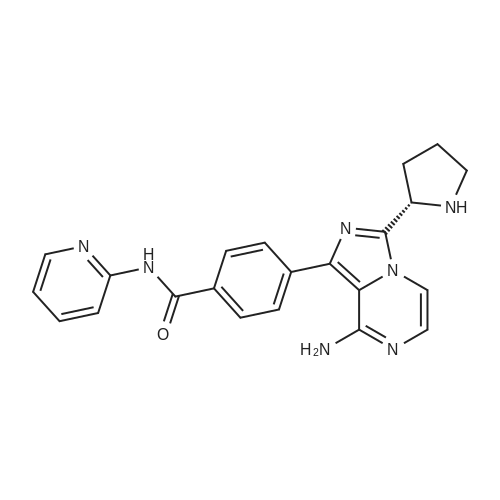
A mixture of compound XI (4.14 g, 10 mmol) , compound IV (2.66 g, 11 mmol), dioxane (34 mL) and K2CO3 aqueous solution (4.14 g K2CO3 in 15 mL water) was added Pd(dppf)Cl2 (73 mg, 0.1 mmol) under nitrogen. The mixture was stirred for 3 h at 90~100 C. The organic phase was separated and concentrated. The residue was purified by silica gel column chromatography using heptane/EtOAc to afford compound XII (4.9 g, 92% yield). (0115) To a round-bottom flask was added compound XII (2.4 g), acetic acid (12 mL) and HBr (33% in acetic acid, 12 mL). The mixture was stirred for 2 h at 20~30 C. Water (300 mL) and DCM (100 mL) was added. The aqueous phase was separated and washed with DCM (100 mL). The aqueous phase was adjusted to pH > 10 with 30% NaOH aqueous solution and extracted with DCM (150 mL). The DCM phase was concentrated to give compound XII I (1.64 g, 91% yield). To a round-bottom flask was added compound XIII (0.50 g, 1.25 mmol), 2-butynoic acid (0.11 g, 1.31 mmol), HATU (0.48 g, 1.25 mmol), DCM (10 mL) and triethylamine (0.50 g, 5 mmol). The mixture was stirred for 3 h at 20~30 C. The reaction mixture was washed with water (5 mL) and concentrated. The residue was purified by silica gel column chromatography using DCM/MeOH to afford compound XV (0.5 g, 90% yield). (0116) XH NMR (400 MHz, DMSO) delta 10.82 (s, 1H), 8.42 - 8.39 (m, 1H), 8.26 - 8.15 (m, 3H), 7.90 - 7.73 (m, 4H), 7.21 - 7.11 (m, 2H), 6.25 - 6.05 (m, 2H), 5.75 - 5.40 (m, 1H), 3.90 - 3.55 (m, 2H), 2.47 - 2.20 (m, 2H), 2.20 - 2.10 (m, 1H), 2.07 - 1.90 (m, 3H), 1.63 (s, 1H). |
| 74% |
With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine; In dichloromethane;Large scale; |
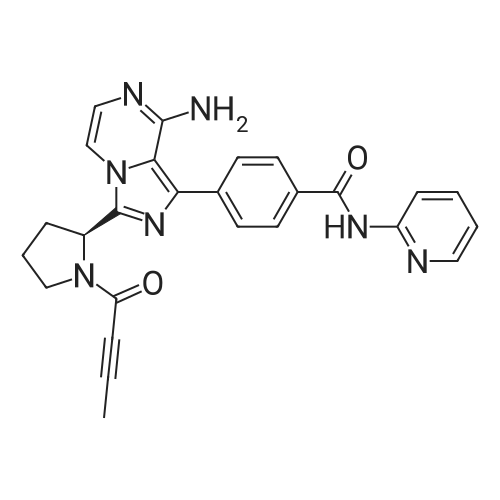
4-{ 8-Amino-3-[(2S)-2-pyrrolidinyl]imidazo[l,5-a]pyrazin-l-yl}-/V-(2-pyridinyl)- benzamide (Compound (VII), 131.7 kg, 1.0 mol. eq.) was slurried in dichloromethane (955 L, 7.25 rel. vol.) and triethylamine (90.1 kg, 2.7 mol. eq.). 2-butynoic acid (33.3 kg, 1.2 mol. eq.) in dichloromethane (263.4 L, 2.0 rel. vol.) was added, followed by l-propylphosphonic acid anhydride (T3P) (50%w/w solution in dichloromethane, 209.8 kg, 1.0 mol. eq.). The resulting organic solution of the product was washed twice with water (658.5 L, 5.0 rel. vol.) and then water (1317 L, 10.0 rel. vol.) was added. The mixture was then acidified using 6M aqueous hydrochloric acid to approximately pH 2.2 and then 2M aqueous hydrochloric acid added to reach a pH of 1.8 to 2.2 before separating the organic phase, which was discarded. Dichloromethane (1317 L, 10.0 rel. vol.) was added to the aqueous phase and the mixture is adjusted to a pH of 4.5 to 5.0 with triethylamine. The organic phase was separated off and the aqueous phase was re-extracted with dichloromethane (527 L, 4.0 rel. vol.). The combined dichloromethane extracts were screened and the organic phase was concentrated to approximately 5.0 rel. vol. Ethanol (1712 L, 13.0 rel. vol.) was added and the mixture distilled (at about 360 mbar) maintaining a constant volume (of 18.0 rel. vol.) by the addition of ethanol (1580 L, 12.0 rel. vol.). A portion of crystalline 4-{8-amino- 3-[(2S)-l-(but-2-ynoyl)pyrrolidin-2-yl]imidazo[l,5-a]pyrazin-l-yl}-/V-(pyridin-2-yl)benzamide (Compound (VIII), 1.32 kg, 0.01 rel. wt.) was added as seed, and the solution held at 50C for 10 hours to crystallize the product. The mixture was then cooled over 7 hours and filtered. The product was washed twice with ethanol (527 L, 4.0 rel. vol.) and then dried at 50C under vacuum to yield a white crystalline solid acalabrutinib (Compound VIII, 113.6 kg, 74%). (0440) [00228] This compound exists as a mixture of conformers in solution and resonances are quoted for the major conformer only. 1H NMR (500 MHz, DMSO-d6) d 1.95-2.02 (m, 4H), 2.09- 2.15 (m, 1H), 2.23-2.38 (m, 2H), 3.81 (t, J = 6.7 Hz, 2H), 5.47 (dd, J = 7.6, 4.3 Hz, 1H), 6.13 (br s, 2H), 7.11 (d, J = 5.1 Hz, 1H), 7.17 (ddd, J = 7.4, 4.8, 0.8 Hz, 1H), 7.70-7.73 (m, 2H), 7.78 (d, J = 5.1 Hz, 1H), 7.82-7.87 (m, 1H), 8.13-8.16 (m, 2H), 8.20-8.23 (m, 1H), 8.39 (ddd, J = 4.8, 1.9, 0.8 Hz, 1H), 10.83 (s, 1H). 13C NMR (126 MHz, DMSO-d6) d 3.3, 23.9, 31.2, 48.2, 51.3, 74.3, 88.3, 107.0, 113.8, 114.7, 119.8, 127.9, 128.3, 129.0, 132.7, 133.2, 137.9, 138.1, 141.0, 148.0, 151.4, 151.8, 152.2, 165.7. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +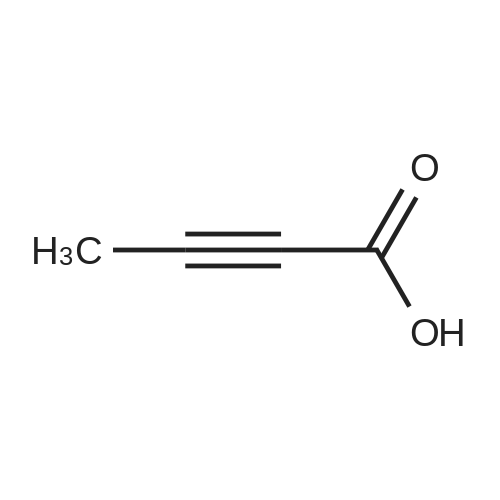

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping