| 81% |
potassium carbonate; In acetone; |
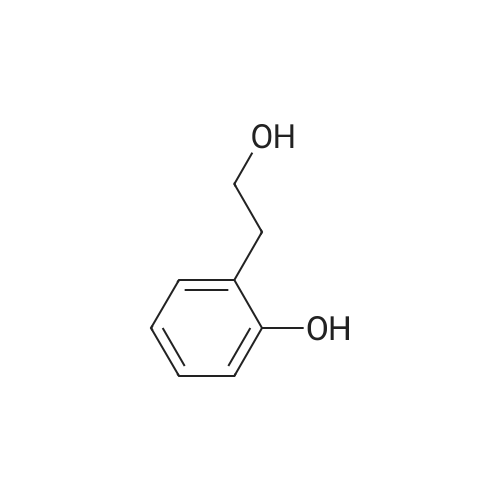
EXAMPLE 27 2-Hydroxyphenethyl alcohol was reacted with bromoacetonitrile in the presence of potassium carbonate to protect the phenolic oxygen, as in Tetrahedron Letters, 1993, 34, 7567-7568. 2-Hydroxyphenethyl alcohol was dissolved in 20 mL acetone. To this was added 1.2 g potassium carbonate. To the stirring mixture was added 0.87 g bromoacetonitrile under nitrogen. The mixture was stirred overnight. The mixture was filtered, and the filtrate was concentrated. The product was purified by flash column chromatography on silica gel, eluding with 1/1 ethyl acetate:hexanes, to yield 81percent of 2-(o-cyanomethyl)phenethyl alcohol. 1H NMR (CD2Cl2): 2.81 (t, 2H), 3.72 (t, 2H), 4.77 (s, 2H), 6.92 (dd, 2H), 7.18 (d, 2H). |
| 81% |
With potassium carbonate; In acetone; |
2-Hydroxyphenethyl alcohol was reacted with bromoacetonitrile in the presence of potassium carbonate to protect the phenolic oxygen, as in Tetrahedron Letters,1993, 34, 7567-7568. 2-Hydroxyphenethyl alcohol was dissolved in 20 mL acetone. To this was added 1.2 g potassium carbonate. To the stirring mixture was added 0.87 g bromoacetonitrile under nitrogen. The mixture was stirred overnight. The mixture was filtered, and the filtrate was concentrated. The product was purified by flash column chromatography on silica gel, eluting with 1/1 ethyl acetate:hexanes, to yield 81percent of 2-(o-cyanomethyl)phenethyl alcohol.1H NMR (CD2Cl2): 2.81 (t, 2H), 3.72 (t, 2H), 4.77 (s, 2H), 6.92 (dd, 2H), 7.18 (d, 2H). 2-(o-Cyanomethyl)phenethyl alcohol (1.0 g, 6.3 mmol) was dissolved in 5 mL anhydrous DMF and added to a stirring solution of sodium hydride (0.25g, 10.4 mmol) in DMF (20 mL). After hydrogen evolution ceases, methyl iodide (0.47 mL, 7.5 mmol) was added dropwise. The mixture was stirred at room temperature under nitrogen for five hours. After aqueous workup, the product was purified using flash column chromatography on silica gel, eluting with 1/5 ethyl acetate/hexanes solvent mixture to yield 0.56 g (56percent) of the desired product, 2-(o-cyanomethyl)phenethyl methyl ether. 1H NMR (CD2Cl2): 2.96 (t, 2H), 3.36 (s, 3H), 3.60 (t, 2H), 4.86 (s, 2H), 7.04 (dd, 2H), 7.31 (d, 2H). 2-(o-Cyanomethyl)phenethyl methyl ether was deprotected following the procedure described in Tetrahedron Letters, 1993, 34, 7567-7568. 2-(o-Cyanomethyl)phenethyl methyl (0.56 g, 3.13 mmol) was dissolved in 40 mL anhydrous ethanol. Platinum dioxide (20 mg) was added to this solution. The solution was purged with hydrogen for 10 minutes, and then stirred under hydrogen overnight. The mixture was filtered, and the filtrate was concentrated. The residue was redissolved in ether, washed with water, and dried over MgSO4. After concentration, 0.39 g (82percent) of 2-hydroxyphenethyl methyl ether was isolated. 1H NMR (CD2Cl2): 2.78 (t, 2H), 3.32 (s, 3H), 3.60 (t, 2H), 2-Hydroxyphenethyl methyl ether was reacted with diethylphosphoramidous dichloride to yield the corresponding phosphorous amidite in the same manner as described for Example 25. 31P NMR (toluene): 137 ppm. The phosphoroamidite was treated with 1M HCl solution following the procedure described for Example 25 to yield the corresponding phosphorochloridite. 31P NMR (toluene): 165 ppm. The phosphochloridite was then reacted with di(2-tolyl)-2,2'-dihydroxy-1,1'-binaphthalene-3,3'-dicarboxylate in the same manner as described for Example 19. 31P NMR (toluene): 125 (major), 127 (minor), 142 (minor). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +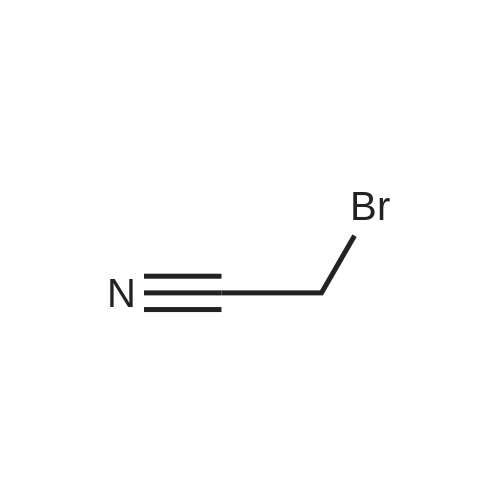

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping