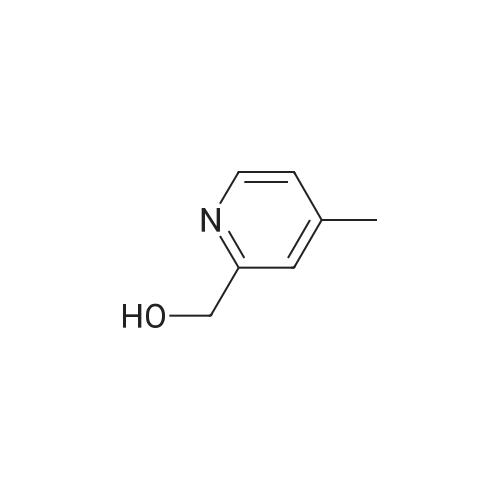
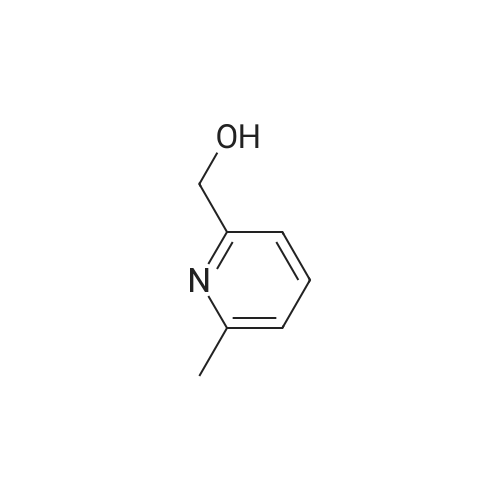
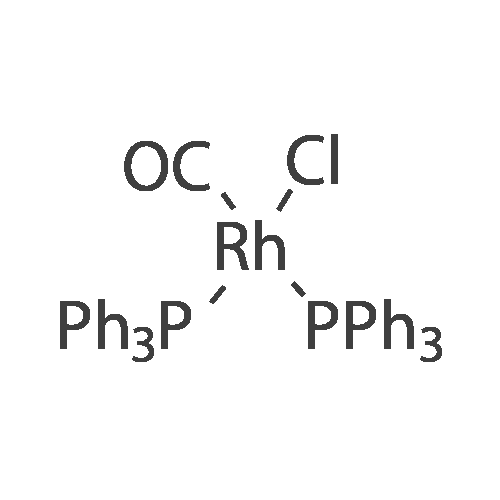| 84% |
With potassium hydroxide In acetonitrile at 35 - 40℃; for 18 h; |
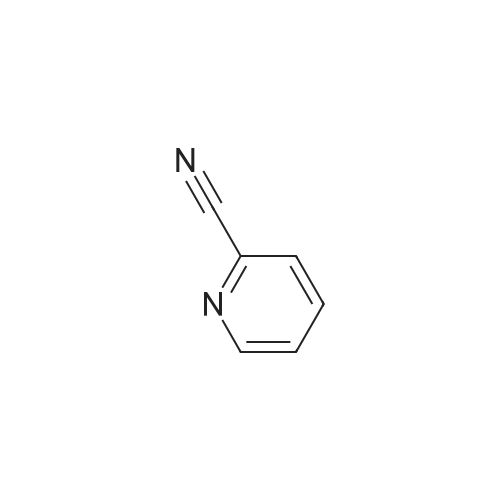
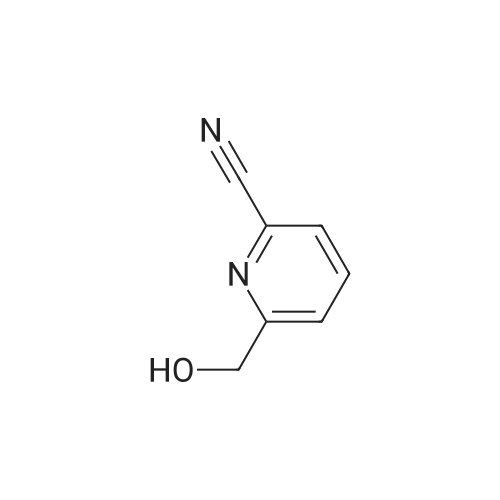
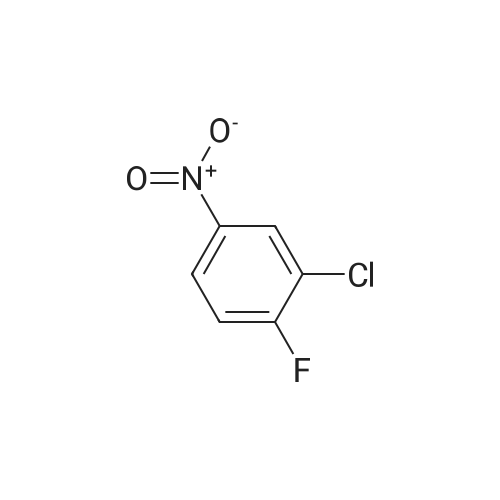
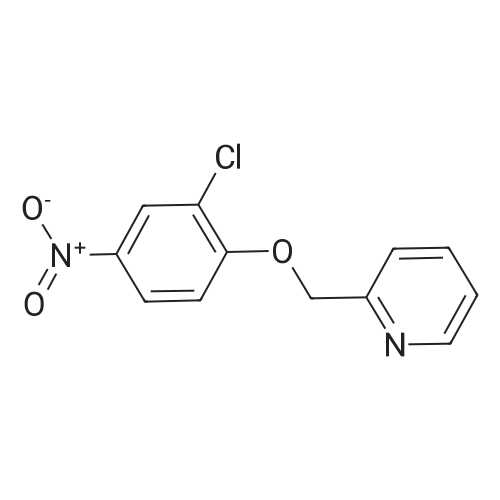
Example 3 Preparation of N-[3-Chloro-4-(2-pyridinylmethoxy)]phenyl-2-cyanoacetamide In a 12-L multi-necked flask, 2-pyridyl carbinol (0.13 kg, 1.19 mole, 1.05 eq) was dissolved in acetonitrile (0.88 L) and to it was added potassium hydroxide flakes (85percent) (80 g, 1.25 eq). The resulting suspension was warmed to 35° C. A solution of 3-chloro-4-fluoronitrobenzene (0.20 kg, 1.14 mol) in acetonitrile (1.0 L) was added at 35-40° C. The mixture was held for 18 h until reaction completion. The mixture was then cooled back to 20-25° C., quenched with water (4 L) and the resulting slurry was filtered and washed with water (3.x.200 mL). The resulting product was isolated as a tan solid (251 g, 84percent yield). A mixture of 3-chloro-4-(2-pyridylmethoxy)nitrobenzene (0.149 kg, 0.56 mole) and 2percent (w/w) of 5percent Pt/C (6.0 g, 50percent water wet) in tetrahydrofuran (0.895 L) was hydrogenated in a 2-L stainless steel Parr reactor at 25 psi, 25° C. for a minimum of 8 h. The mixture was filtered through a celite pad (50 g, 15 cm diameter) and washed with tetrahydrofuran (0.45 L). The filtrate was distilled to a volume of 0.30 L and the concentrate was transferred to a 2-L multi-neck flask and used as is in the next step. To the 2-L flask equipped with mechanical stirrer, temperature probe, claisen head and condenser was added ethylcyanoacetate (0.421 kg, 3.72 mole, 6.6 eq.). The reaction mixture was heated to (100-115° C.) while removing tetrahydrofuran and ethanol. The temperature was raised to 125° C. and the mixture was held for a minimum of 24 h until the aniline starting material was consumed and no distillate was collected. The mixture was cooled to room temperature over 1 h. At 50-60° C., solids crystallized out and ethyl acetate (0.15 L) was added. The mixture was further cooled to 0-10° C. and held for 1 h. The mixture was filtered on a 15 cm diameter Buchner funnel and washed with 50 mL of the filtrate followed by pre-cooled (0-10° C.) ethyl acetate (0.15 L). The product was dried at 60° C. for a minimum of 16 h in a vacuum oven to give the titled compound (0.12 kg, 71percent) as a brown solid. The product was purified by slurrying in cold ethyl acetate (1-1.3 volumes) for 1 hr. 1H NMR: δ (DMSO-d6) 10.31 (s, 1H, NH), 8.58 (dd, 1H, Ar), 7.86 (dt, 1H, Ar), 7.75 (d, 1H, Ar), 7.55 (d, 1H, Ar), 7.39-7.32 (m, 2H, Ar), 7.21 (d, 1H, Ar), 5.25 (s, 2H, OCH2Pyr), 3.88 (s, 2H, NCCH2CO). |
| 81.9% |
With potassium carbonate In N,N-dimethyl-formamide at 20 - 40℃; for 32 h; |
Synthesis of 3-chloro-4-(2-pyridylmethoxy)nitrobenzene 2-pyridinyl carbinol (31.08 g, 1.05 eq) was dissolved in ACN (750 mL) and KOH flakes (85percent) were added (20.6 g, 1.25 eq.). The resulting suspension was warmed to 35° C. A solution of the 3-chloro-4-fluoronitrobenzene (50.0 g, 0.285 mol) in ACN (250 mL) was added at 35-40° C. The mixture was held for 14 hours. The mixture was then cooled back to 20-25° C., quenched with H2O (1 L) and the resulting slurry filtered and washed with H2O (3.x.100 mL). The resulting product was isolated as a tan solid in 93percent yield with a greater than 99.5percent purity as determined by HPLC area.; Experimental results for the reaction of Example 1 with different bases and solvents are shown in Table 1. The last three entries on Table 1 are large scale runs in which a 5percent excess of pyridyl carbinol was used. TABLE 1 Preparation of Nitroaryl Intermediate Scale Vol- Base Time Temp Yield Purity (g) Solvent umes Base Eq. (h) (° C.) (percent) (percent) 2.0 DMF 20 KOH 1.1 20 RT 90.5 94.7 2.0 NMP 10 NaH 1.2 20 RT 48.7 78.4 2.0 ACN 20 KOH 1.1 4 RT 93.2 98.4 2.0 EtOAc 10 KOH 1.1 72 RT NA NA 10.0 DMF 15 KOH 1.1 23 RT 76.5 96.7 4 35 10.0 ACN 15 KOH 1.1 23 RT 91.8 99.4 2.0 THF 20 KOH 1.1 20 RT 87.5 99.2 2.0 DMF 20K2CO3 1.0 26 RT 81.9 98.5 extra 3 40 2.0eqK2CO3 3 40 2.0 ACN 20K2CO3 1.0 18 RT NA NA 3 40 2.0 THF 20K2CO3 1.0 18 RT NA NA 50.0 ACN 20 KOH 1.1 20 40 93.5 99.8 200 ACN 20 KOH 1.1 16 40 86.0 97.6 200 ACN 20 KOH 1.25 16 40 93.5 96.9 400 ACN 20 KOH 1.25 16 40 91.5 98.4 400 ACN 20 KOH 1.25 16 40 93.8 98.1 NA = not applicable RT = room temperature (20-25° C.) |
| 76.5% |
With potassium hydroxide In N,N-dimethyl-formamide at 20 - 35℃; for 20 - 27 h; |
Synthesis of 3-chloro-4-(2-pyridylmethoxy)nitrobenzene 2-pyridinyl carbinol (31.08 g, 1.05 eq) was dissolved in ACN (750 mL) and KOH flakes (85percent) were added (20.6 g, 1.25 eq.). The resulting suspension was warmed to 35° C. A solution of the 3-chloro-4-fluoronitrobenzene (50.0 g, 0.285 mol) in ACN (250 mL) was added at 35-40° C. The mixture was held for 14 hours. The mixture was then cooled back to 20-25° C., quenched with H2O (1 L) and the resulting slurry filtered and washed with H2O (3.x.100 mL). The resulting product was isolated as a tan solid in 93percent yield with a greater than 99.5percent purity as determined by HPLC area.; Experimental results for the reaction of Example 1 with different bases and solvents are shown in Table 1. The last three entries on Table 1 are large scale runs in which a 5percent excess of pyridyl carbinol was used. TABLE 1 Preparation of Nitroaryl Intermediate Scale Vol- Base Time Temp Yield Purity (g) Solvent umes Base Eq. (h) (° C.) (percent) (percent) 2.0 DMF 20 KOH 1.1 20 RT 90.5 94.7 2.0 NMP 10 NaH 1.2 20 RT 48.7 78.4 2.0 ACN 20 KOH 1.1 4 RT 93.2 98.4 2.0 EtOAc 10 KOH 1.1 72 RT NA NA 10.0 DMF 15 KOH 1.1 23 RT 76.5 96.7 4 35 10.0 ACN 15 KOH 1.1 23 RT 91.8 99.4 2.0 THF 20 KOH 1.1 20 RT 87.5 99.2 2.0 DMF 20K2CO3 1.0 26 RT 81.9 98.5 extra 3 40 2.0eqK2CO3 3 40 2.0 ACN 20K2CO3 1.0 18 RT NA NA 3 40 2.0 THF 20K2CO3 1.0 18 RT NA NA 50.0 ACN 20 KOH 1.1 20 40 93.5 99.8 200 ACN 20 KOH 1.1 16 40 86.0 97.6 200 ACN 20 KOH 1.25 16 40 93.5 96.9 400 ACN 20 KOH 1.25 16 40 91.5 98.4 400 ACN 20 KOH 1.25 16 40 93.8 98.1 NA = not applicable RT = room temperature (20-25° C.) |
| 48.7% |
With sodium hydride In 1-methyl-pyrrolidin-2-one at 20 - 25℃; for 20 h; |
Synthesis of 3-chloro-4-(2-pyridylmethoxy)nitrobenzene 2-pyridinyl carbinol (31.08 g, 1.05 eq) was dissolved in ACN (750 mL) and KOH flakes (85percent) were added (20.6 g, 1.25 eq.). The resulting suspension was warmed to 35° C. A solution of the 3-chloro-4-fluoronitrobenzene (50.0 g, 0.285 mol) in ACN (250 mL) was added at 35-40° C. The mixture was held for 14 hours. The mixture was then cooled back to 20-25° C., quenched with H2O (1 L) and the resulting slurry filtered and washed with H2O (3.x.100 mL). The resulting product was isolated as a tan solid in 93percent yield with a greater than 99.5percent purity as determined by HPLC area.; Experimental results for the reaction of Example 1 with different bases and solvents are shown in Table 1. The last three entries on Table 1 are large scale runs in which a 5percent excess of pyridyl carbinol was used. TABLE 1 Preparation of Nitroaryl Intermediate Scale Vol- Base Time Temp Yield Purity (g) Solvent umes Base Eq. (h) (° C.) (percent) (percent) 2.0 DMF 20 KOH 1.1 20 RT 90.5 94.7 2.0 NMP 10 NaH 1.2 20 RT 48.7 78.4 2.0 ACN 20 KOH 1.1 4 RT 93.2 98.4 2.0 EtOAc 10 KOH 1.1 72 RT NA NA 10.0 DMF 15 KOH 1.1 23 RT 76.5 96.7 4 35 10.0 ACN 15 KOH 1.1 23 RT 91.8 99.4 2.0 THF 20 KOH 1.1 20 RT 87.5 99.2 2.0 DMF 20K2CO3 1.0 26 RT 81.9 98.5 extra 3 40 2.0eqK2CO3 3 40 2.0 ACN 20K2CO3 1.0 18 RT NA NA 3 40 2.0 THF 20K2CO3 1.0 18 RT NA NA 50.0 ACN 20 KOH 1.1 20 40 93.5 99.8 200 ACN 20 KOH 1.1 16 40 86.0 97.6 200 ACN 20 KOH 1.25 16 40 93.5 96.9 400 ACN 20 KOH 1.25 16 40 91.5 98.4 400 ACN 20 KOH 1.25 16 40 93.8 98.1 NA = not applicable RT = room temperature (20-25° C.) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping