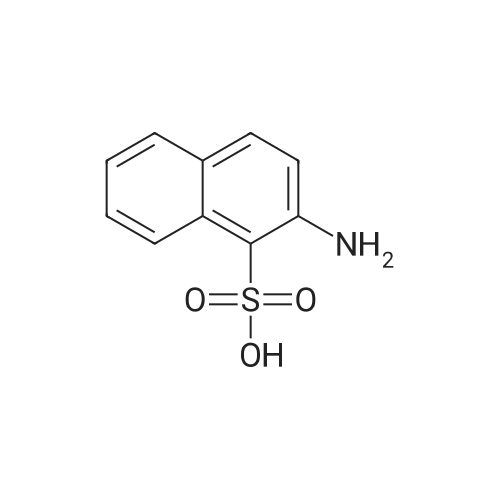
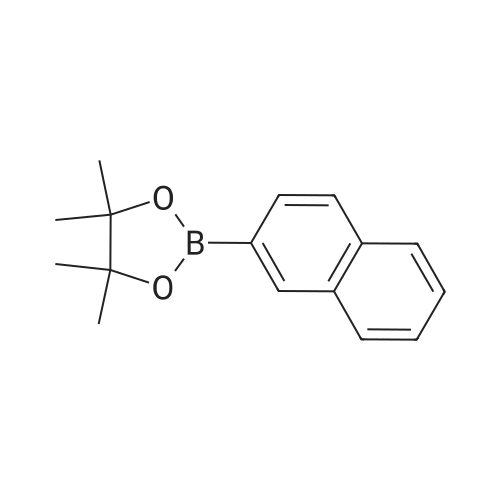
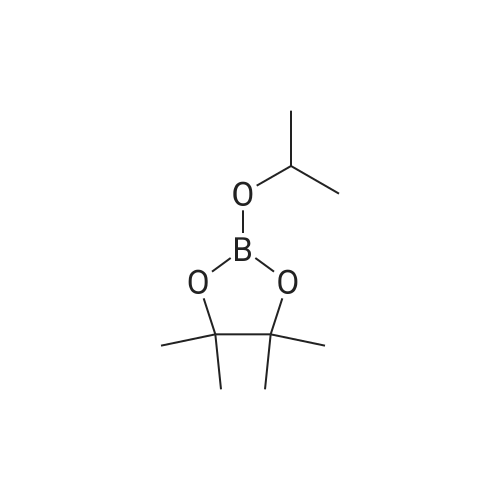
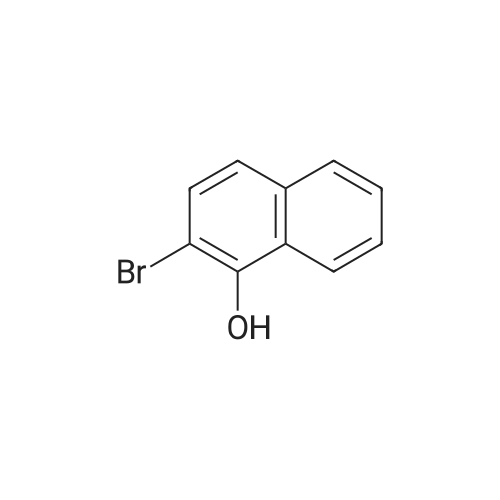
| 75% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In toluene; for 24h; |
Sub 2-1-3 (4.14g, 20mmol) was dissolved in toluene, bis-pinacolato diboron (5.58g, 22mmol), Pd(dppf)Cl2 catalyst (0.44g, 0.6mmol), KOAc (5.89g, 60mmol )was added. A borate compound was synthesized by stirring for 24 hours after the addition, as, after the thus obtained compound was separated over a silicagel column and recrystallized to obtain 3.8g of Sub 2 (3). (Yield: 75%) |
| 61% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 120℃; for 8h;Inert atmosphere; |
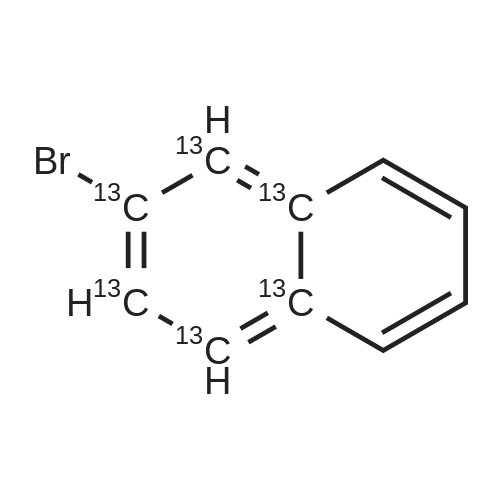
Fill a dry 500mL double-neck round bottom flask with nitrogen.Add 2-naphthalene bromide (20.6g, 0.1mol),Bis (pinacolato) diboron (25.4g, 0.1mol),Potassium acetate (19.6g, 0.2mol),[1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium (II) (0.005mol),1,4-dioxane (200 mL), reacted at 120 C for 8 hours;After the reaction was completed, it was cooled to room temperature, quenched with water, and extracted with dichloromethane (three times with 200 mL extraction). The extract was dried over anhydrous magnesium sulfate and spin-dried.The crude product was separated by silica gel column chromatography.2-naphthoboronic acid pinacol ester (15.5 g, yield 61%) was obtained. |
| 47% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,2-dimethoxyethane; for 4h;Inert atmosphere; Reflux; |
2eBromonaphthalene (299 mg, 1.14 mmol) KAcO (416 mg,4.24 mmol) and bis(pinacolato)diboron (558 mg,2.20 101 mmol) were dissolved in 50 mL of 1,2-dimethoxyethane (DME) and the mixture was purged with N2 gas. [1,10-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)complex with dichloromethane (Pd(dppf)Cl2CH2Cl2) (49 mg,5.94 102 mmol)was added to the resulting solution and refluxedfor 4 h. The mixture was extracted with ethyl acetate. The solutionwas washed withwater, and passed through phase separator paper.After the solvent was removed, the crude mixture was purified bysilica gel column chromatography (ethyl acetate:hexane 1:40) togive 1 as a yellow solid (170 mg, yield: 47%). 1H NMR (400 MHz,CDCl3): delta 8.37 (s, 1H), 7.89 (d, J 7.6 Hz 3H), 7.86e7.79 (m, 3H), 1.39(s, 12H). HRMS (ESI-TOF) calculated for C16H19BO2 [MNa] :276.1421, found: 276.1407. |
|
With bis-triphenylphosphine-palladium(II) chloride; potassium acetate; In 1,4-dioxane; at 20 - 80℃;Inert atmosphere; |
General procedure: The appropriate 2-iodothiophene (100mg,0.47mmol), bis(pinacolato)diboron(137mg, 0.57mmol), andbis(triphenylphosphine)palladium (II) dichloride (33.4mg,0.04mmol) were dissolved in dry 1,4-dioxane in an inertatmosphere. Potassium acetate (93.4mg,0.9521mmol) was then added at room temperature, and the mixturewas heated at 80C for 5-6 h. After solvent evaporation, water was added to theresidue extracted with ethyl acetate. The organic layer was washed with brine,and dried by distillation to obtain a crude product, which was used withoutfurther purification. Other aryl halide, 2-bromonaphthalene andbromoahthracene, also followed same procedure with 2-iodothiophene. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping