| 75% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 18h; |
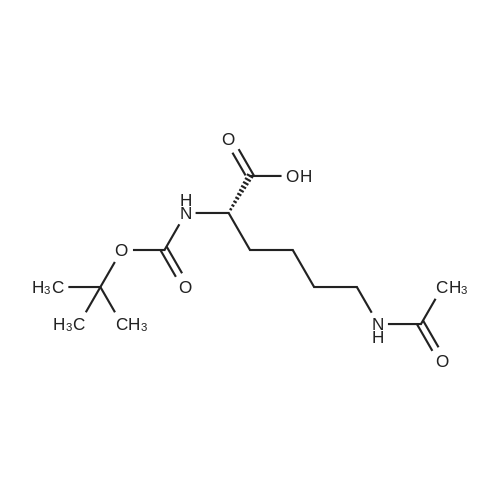
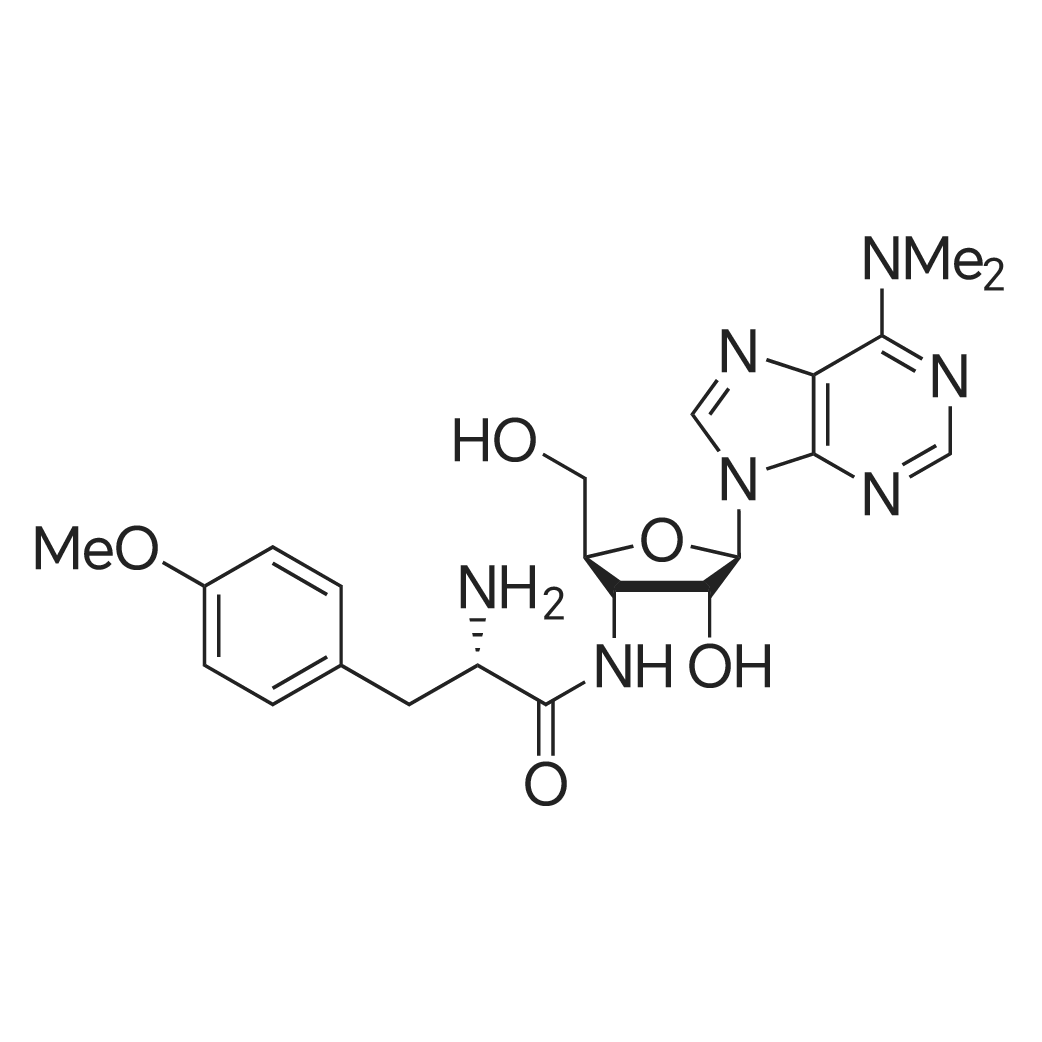
A mixture of puromycin dihydrochloride (50 mg, 0.092 mmol) , Oi-Boc-Lys (epsilon-Ac) -OH (31 mg, 0.11 mmol) , EDC-HC1 (21 mg, 0.11 mmol), HOBt-H20 (17 mg, 0.11 mmol), and DIEA (N,N- diisopropylethylamine) (18 ]1L, 0.11 mmol) was stirred in DMF (10 ml) at rt for 18 h. After concentrating the solution under reduced pressure, the residue was dissolved in DCM. The DCM solution was washed with 0 three times, dried over Na2S04, and concentrated. The crude oil was purified by silica gel column chromatography using a linear gradient from 5 to 10 % MeOH in DCM and dried to yield a-Boc-Lys ( E-AC) -Puromycin as a white solid (55 mg, 75 % yield) : mp: 182-183 C; NMR (500 MHz, DMSO) delta 8.43 (s, 1H, H-29) , 8.22 (s, 1H, H-32), 8.15 (d, J = 1.1 Hz, 1H, H-8'), 7.74 (d, J = 7.1 Hz, 2H, Eta-2', H-23'), 7.15 (d, J = 8.4 Hz, 2H, H-17), 6.86 (d, J = 8.3 Hz,' 1H, H-9'), 6.80 (d, J = 8.6 Hz, 2H, H-16, H-20), 6.03 (d, J = 4.6 Hz, 1H, H-28), 5.98 (d, J = 2.8 Hz, 1H, H-27) , 5.18 (t, J = 5.4 Hz, 1H, H-22), 4.60 (dd, J - 13.6, 8.1 Hz, 1H, H-7), 4.52 - 4.39 (m, 2H, H-25, H-26), 3.98 - 3.76 (m, 2H, H-24), 3.70 (s, 3H, H-14) , 3.69 -3.40 (m, 6H, H-34, H-35), 3.02 - 2.86 (m, 3H, H-3, H-21) , 2.76 (dd, J = 13.7, 8.7 Hz, 1H, H-21), 1.77 (s, 3H, H-l) , 1.52 - 1.38 (m, 2H, H-6), 1.38 - 1.32 (s, 9H, H-ll, H-12, H-13), 1.33 - 1.05 (ra, 6H, H-4, H-5) ppm. 13C NMR (101 MHz, DMSO) delta 169.5 (C-8, C-23), 158.4 (C-9), 155.8 (C-2), 154.8 (C-33), 152.4 (C-32), 150.2 (C-31) , 138.4 (C-29), 138.3 (C-15), 131.0 (C-17, C-19), 129.8 (C-18) , 120.2 (C- 30), 114.0 (C-16, C-20), 90.0 (C-28) , 78.8 (C-10, C-25) , 73.8 (C- 27), 61.5 (C-24), 55.6 (C-26) , 55.3 (C-7), 54.5 (C-22), 51.0 (C- 14), 40 ( (C-34, C-35, underneath DMSO peak), 39.1 (C-3), 38.2 (C- 21), 32.5 (C-6), 29.5 (C-4) , 28.9 (C-ll, C-12, C-13), 23.7 (C-5) , 23.4 (C-l); MS (ifl/z) : [M] + calcd. for C35H51N9O9, 742.38; found, 742.47. |
| 75% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 18h; |
A mixture of 15 puromycin dihydrochloride (50 mg, 0.092 mmol), 16 alpha-Boc-Lys(epsilon-Ac)-OH (31 mg, 0.11 mmol), 17 EDC.HCl (21 mg, 0.11 mmol), HOBt.H2O (17 mg, 0.11 mmol), and 18 DIEA (N,N-diisopropylethylamine) (18 muL, 0.11 mmol) was stirred in 19 DMF (10 ml) at rt for 18 h. After concentrating the solution under reduced pressure, the residue was dissolved in 20 DCM. The DCM solution was washed with H2O three times, dried over Na2SO4, and concentrated. The crude oil was purified by silica gel column chromatography using a linear gradient from 5 to 10% 21 MeOH in DCM and dried to yield alpha-Boc-Lys(s-Ac)-Puromycin as a white solid (55 mg, 75% yield): mp: 182-183 C.; 1H NMR (500 MHz, DMSO) delta 8.43 (s, 1H, H-29), 8.22 (s, 1H, H-32), 8.15 (d, J=7.7 Hz, 1H, H-8?), 7.74 (d, J=7.1 Hz, 2H, H-2?, H-23?), 7.15 (d, J=8.4 Hz, 2H, H-17), 6.86 (d, J=8.3 Hz, 1H, H-9?), 6.80 (d, J=8.6 Hz, 2H, H-16, H-20), 6.03 (d, J=4.6 Hz, 1H, H-28), 5.98 (d, J=2.8 Hz, 1H, H-27), 5.18 (t, J=5.4 Hz, 1H, H-22), 4.60 (dd, J=13.6, 8.1 Hz, 1H, H-7), 4.52-4.39 (m, 2H, H-25, H-26), 3.98-3.76 (m, 2H, H-24), 3.70 (s, 3H, H-14), 3.69-3.40 (m, 6H, H-34, H-35), 3.02-2.86 (m, 3H, H-3, H-21), 2.76 (dd, J=13.7, 8.7 Hz, 1H, H-21), 1.77 (s, 3H, H-1), 1.52-1.38 (m, 2H, H-6), 1.38-1.32 (s, 9H, H-11, H-12, H-13), 1.33-1.05 (m, 6H, H-4, H-5) ppm. 13C NMR (101 MHz, DMSO) delta 169.5 (C-8, C-23), 158.4 (C-9), 155.8 (C-2), 154.8 (C-33), 152.4 (C-32), 150.2 (C-31), 138.4 (C-29), 138.3 (C-15), 131.0 (C-17, C-19), 129.8 (C-18), 120.2 (C-30), 114.0 (C-16, C-20), 90.0 (C-28), 78.8 (C-10, C-25), 73.8 (C-27), 61.5 (C-24), 55.6 (C-26), 55.3 (C-7), 54.5 (C-22), 51.0 (C-14), 40 ((C-34, C-35, underneath DMSO peak), 39.1 (C-3), 38.2 (C-21), 32.5 (C-6), 29.5 (C-4), 28.9 (C-11, C-12, C-13), 23.7 (C-5), 23.4 (C-1); MS (m/z): [M]+ calcd. for C35H51N9O9, 742.38; found, 742.47. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping