|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;dmap; In acetonitrile; at 20℃; for 16h; |
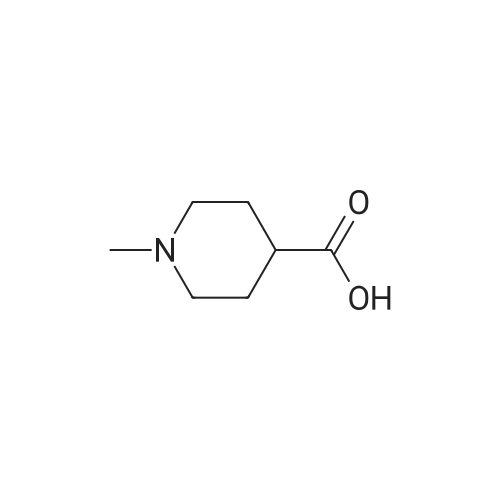
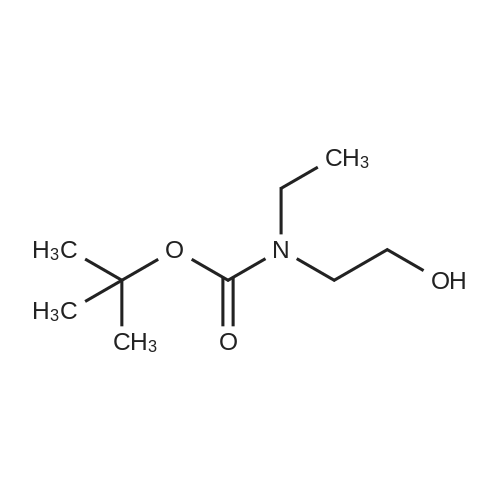
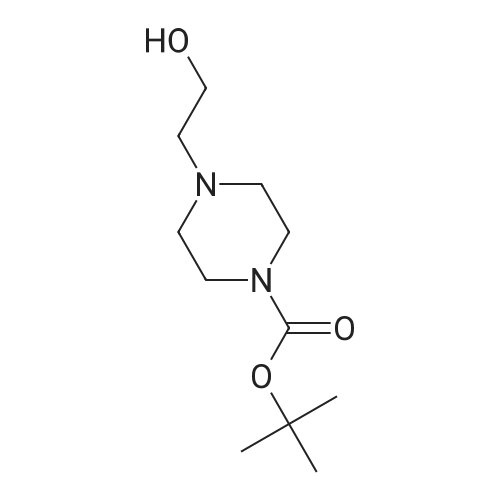
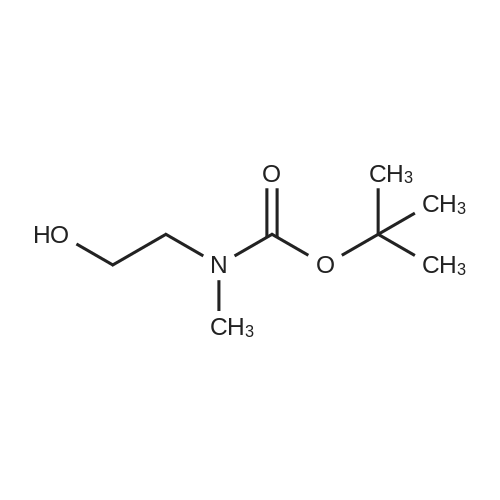
A mixture of ethyl piperidine-4-carboxylate (4.72 g), methyl iodide (2.24 mL), potassium carbonate (8.29 g) and acetonitrile (50 mL) was stirred at room temperature for 2 hrs. The reaction mixture was concentrated under reduced pressure and water (150 mL) was added. The mixture was extracted with ethyl acetate (150 mL). The ethyl acetate layer was washed with saturated brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. A 1N aqueous sodium hydroxide solution (20 mL) was added to the residue (2.64 g), and the mixture was stirred overnight at room temperature. The reaction mixture was neutralized by adding 1N hydrochloric acid (20 mL) and the mixture was concentrated under reduced pressure. Ethanol was added to the residue, and the precipitate was filtered off. The filtrate was concentrated under reduced pressure. This step was repeated and ethanol and ethyl acetate were added to the residue for crystallization to give 1-methylpiperidine-4- carboxylic acid (1.79 g) as a colorless solid. H-NMR (CD30D) : 1.80-1. 98 (2H, m), 2.00-2. 14 (2H, m), 2.28- 2.42 (lH, m), 2.78 (3H, s), 2.88-3. 04 (2H. m), 3.32-3. 44 (2H. m). A mixture of <strong>[68947-43-3]1-methylpiperidine-4-carboxylic acid</strong> (1.72 g) obtained above, tert-butyl 2-hydroxyethyl (methyl) carbamate (1.75 g) obtained in Reference Example 1, [1-ETHYL-3- [3-] (dimethylamino) propyl] carbodiimide hydrochloride (2.30 g), 4- dimethylaminopyridine (0.24 g) and acetonitrile (50 mL) was stirred at room temperature for 16 hrs. The reaction mixture was concentrated under reduced pressure and a saturated aqueous sodium hydrogen carbonate solution (50 mL) was added to the residue. The mixture was extracted with ethyl acetate (100 [ML).] The ethyl acetate layer was washed with saturated brine (50 [ML),] dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (eluted with ethyl acetate: [HEXANE=50] : 50, then 80: 20). IN Hydrochloric acid (25 mL) was added to the purified product (2.73 g), and the mixture was stirred overnight at room temperature. The reaction mixture was concentrated under reduced pressure and isopropanol was added. The mixture was again concentrated under reduced pressure and the precipitated solid was collected by filtration to give the title compound (1.72 g) as a colorless solid. [H-NMR] [(DMSO-D6)] : 1.70-2. 20 (4H, m), 2.40-3. 50 [(13H,] m), 4. 31 (2H, m), 9.25 (2H, br), 10.77 (lH, br). |
|
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In acetonitrile; at 15 - 30℃; for 16h; |
A mixture of <strong>[68947-43-3]1-methylpiperidine-4-carboxylic acid</strong> (1.72 g) obtained above, tert-butyl 2-hydroxyethyl(methyl)carbamate (1.75 g) obtained in Reference Synthetic Example 1, 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (2.30 g), 4-dimethylaminopyridine (0.24 g) and acetonitrile (50 mL) was stirred at room temperature for 16 hrs. The reaction solution was concentrated under reduced pressure, and to the residue was added saturated aqueous solution of sodium bicarbonate (50 mL), and extracted with ethyl acetate (100 mL). The ethyl acetate layeer was washed with saturated brine (50 mL), and dried over anhydrous magnesium sulfate, followed by concentrating under reduced pressure. The residue was purified with basic silica gel column chromatography (eluted with methanol : ethyl acetate = 50 : 50, then 80 : 20). To the purified material (2.73 g) was added 1 N hydrochloric acid (25 mL), and stirred at room temperature overnight. The reaction solution was concentrated under reduced pressure, and isopropanol was added, then, concentrated again under reduced pressure. The precipitated crystals were collected by filtration to give title compound as colorless solid (1.72 g).1H-NMR (DMSO-d6) : 1.70-2.20(4H,m), 2.40-3.50 (13H,m), 4.31(2H,m), 9.25(2H,br), 10.77 (1H,br). |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;dmap; In acetonitrile; at 20℃; for 16h; |
A mixture of <strong>[68947-43-3]1-methylpiperidine-4-carboxylic acid</strong> (1.72 g) obtained above, tert-butyl 2-hydroxyethyl(methyl)carbamate (1.75 g) obtained in Reference Example 1, 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (2.30 g), 4-dimethylaminopyridine (0.24 g) and acetonitrile (50 mL) was stirred at room temperature for 16 hrs. The reaction mixture was concentrated under reduced pressure and a saturated aqueous sodium hydrogen carbonate solution (50 mL) was added to the residue. The mixture was extracted with ethyl acetate (100 mL). The ethyl acetate layer was washed with saturated brine (50 mL), dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (eluted with ethyl acetate:hexane=50:50, then 80:20). 1N Hydrochloric acid (25 mL) was added to the purified product (2.73 g), and the mixture was stirred overnight at room temperature. The reaction mixture was concentrated under reduced pressure and isopropanol was added. The mixture was again concentrated under reduced pressure and the precipitated solid was collected by filtration to give the title compound (1.72 g) as a colorless solid.1H-NMR(DMSO-d6) : 1.70-2.20 (4H,m), 2.40-3.50 (13H,m), 4.31 (2H,m), 9.25 (2H,br), 10.77 (1H,br). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping